The combination of tungsten metal with carbon atoms results in the material known as tungsten carbide. It is a chemical substance called WC, and its most basic form is a gray powder. It is an extremely hard substance that is frequently utilized in the manufacture of tools and wear-resistant components. It has an extremely high melting point and can resist extremely high temperatures. Tungsten carbide is also extremely corrosion resistant. Compared to other metals like gold, silver, or platinum, tungsten carbide is particularly durable.

Tungsten is a solid, light grey metal with a melting point of 3422 °C and excellent corrosion resistance. Its hardness (7.5 on the Mohs Scale) is equivalent to that of hardened steel or emerald, making it soft enough to be cut with a hacksaw. It’s also quite ductile and can be twisted into wire, which is why tungsten is used as a filament in most lightbulbs. It is also utilized in medical equipment and for specialized welding applications.
Interesting Science Videos
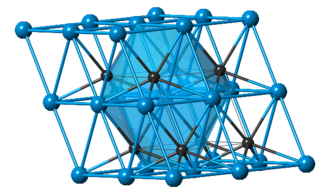
Structure of Tungsten Carbide
- There is a hexagonal form of tungsten carbide as well as a cubic high-temperature form of tungsten carbide with a rock salt structure.
- The hexagonal structure is composed of a simple hexagonal lattice of metal atoms in layers that lie directly over one another, therefore not tightly packed, with carbon atoms occupying half the interstices, giving both tungsten and carbon a conventional trigonal prismatic 6 coordination.
- The tungsten carbon link length is 220 pm, the minimum separation between tungsten atoms in neighboring layers is 284 pm, and the bond lengths between tungsten atoms in a hexagonally packed layer are 291 pm.
- The length of the tungsten-carbon link is therefore equal to the length of a single bond in W(CH3)6 (218 pm), where tungsten is highly twisted, trigonal, and prismatic.
Synthesis of Tungsten Carbide
Tungsten carbide is made by heating tungsten metal and carbon to 1400°C – 2000°C. A lower-temperature fluid bed procedure that combines tungsten metal or blue WO3 with a CO/CO2 mixture and H2 between 900°C and 1200 °C is another method.
Another method of creating WC involves heating WO3 with graphite: either directly at 900 °C or in hydrogen at 670 °C, then carburization in argon at 1000 °C. The following chemical vapor deposition techniques have been researched:
Tungsten hexachloride is reacted with hydrogen (as a reducing agent) and methane (as a carbon source) at 670 °C (943 K).
WCl6 + H2 + CH4 → WC + 6 HClAt 350 °C (623 K), tungsten hexafluoride is reacted with hydrogen (as a reducing agent) and methanol (as a carbon source).
WF6 + 2 H2 + CH3OH → WC + 6 HF + H2OPhysical Properties of Tungsten Carbide
Different grades of tungsten carbide have different strengths, rigidities, and other qualities, however all tungsten carbide material fits into the main categories described below.
- Tungsten carbide has a high melting temperature of 2870°C (5200°F) and a higher boiling temperature of 6000°C (10830°F).
- The density of tungsten carbide is 15.63 g/cm3.
- The stiffness of tungsten carbide compositions is two to three times that of steel and four to six times that of cast iron and brass. Young’s Modulus values can go up to 94,800,000 psi.
- The electrical resistivity of tungsten carbide (approximately 0.2 m) is comparable to that of other metals (for example, vanadium 0.2 m).
- When heated to 1400°F, tungsten carbide retains most of its room temperature hardness. Some grades have the toughness of steels at room temperature when heated to 1400°F.
- Tungsten carbide is also highly corrosion resistant. Because it does not react with most acids or bases, it is appropriate for use in settings where other materials would decay fast.
- Tungsten carbide preserves hardness and impact strength at cryogenic temperatures. (-453°F.)
- A longitudinal wave (sound) traveling through a thin rod of tungsten carbide travels at 6220 m/s.
- The specific heat of tungsten carbide is approximately 50% to 70% that of carbon steel.
- The specific gravity of tungsten carbide is approximately one-half to two times that of carbon steel.
- Thermal conductivity for tungsten carbide is 110 Wm-1K-1 at 1 atm.
Chemical Properties of Tungsten Carbide
- W2C and WC are well-known tungsten and carbon compounds. Both chemicals are found in coatings in various proportions depending on the coating process.
- It is also feasible to synthesize tungsten and carbon meta-stable compounds by heating WC to high temperatures with plasma and then quenching in an inert atmosphere (plasma spheroidization).
- WC particles get spheroidized during this process, becoming WC1-x, which exists in a meta-stable state at room temperature.
- The WC oxidizes at 500-600 °C (773-873 K). Aqueous hydrogen peroxide solutions easily oxidize finely milled WC.
- When heated, WC decomposes into tungsten and carbon, as in high velocity oxygen fuel (HVOF) and high energy plasma (HEP) processes.
- The only acid that may degrade tungsten carbide at temperatures above room temperature is a mixture of hydrofluoric acid and nitric acid (HF/HNO3).
- It is unreactive to dry H2 up to its melting point and reacts with fluorine gas at ambient temperature and chlorine over 400 °C or 752 °F. Finely powdered WC quickly oxidizes in aqueous hydrogen peroxide solutions. It combines with aqueous sodium carbonate at high temperatures and pressures to generate sodium tungstate, which is used to recover scrap cemented carbide due to its selectivity.
Applications of Tungsten Carbide
Due to its distinct characteristics and remarkable attributes, tungsten carbide is a highly sought-after material for several applications. Tungsten has a wide range of uses and is valuable in a variety of industries than only manufacturing and industry. Examples include the medical sector, the fashion industry, and many more.
Construction
Construction requires the use of tools that are both powerful and durable in order to deal with the materials that most constructions are built of. It takes a very strong and resilient blade or drill bit, such those composed of tungsten carbide, to cut through tough materials like cement and asphalt. Due to its nearly indestructible nature, tungsten carbide is frequently used in construction products like drill bits and saws.
Ammunition
Tungsten carbide is widely employed in armor-piercing ammunition, either as a monolithic sintered type or as a tungsten carbide cobalt composite, especially when depleted uranium is unavailable or politically unpalatable. Tungsten carbide was only utilized to make machine tools and a small number of projectiles. It is an effective penetrator due to its great hardness and density.
Alloys
Alloys are made by combining metals with other metals or elements to produce electronics, building materials, industrial gears, and even aviation equipment. These alloys are created to have the particular characteristics, like as strength or heat resistance, required for each unique product and its usage. For building supplies and equipment, tungsten carbide alloys are a particularly well-liked option. These alloys are produced using around 17% of the total tungsten carbide utilized today.
Jewelry
A new and intriguing use for tungsten carbide is in the jewelry industry. Tungsten carbide jewelry may be as beautiful as any other item you might ordinarily wear if it is cut, produced, and polished properly. Tungsten carbide is a more affordable alternative for jewelry than the gold or silver we’re used to paying for and is also recognized for its remarkable and superior scratch resistance. Due to its durability, this metal is now ruling the market and is frequently used to make earrings, necklaces, and rings.

[Image source: tungstenworld]
Surgical Instruments
Many of the prospective applications for tungsten carbide are still being found, and one of these new applications is in the medical arena. Tungsten carbide is frequently used to make surgical equipment because it improves performance and is corrosion-resistant. It increases the durability and strength of the surgical instruments. The surgical sector benefits from the features of tungsten carbide, such as its ability to be sharpened while preserving hardness.
Effects of Tungsten Carbide on Human and Environment
- Tungsten carbide is one of the less hazardous substance and is not carcinogenic. Although inhaling tungsten carbide dust should be avoided, no significant injury to the lungs has been demonstrated thus far.
- Furthermore, tungsten carbide swallowing (oral uptake) is thought to be safe.
- Modern filtration mechanisms restrict the discharge of tungsten carbide nanoparticles into the environment by manufacturing facilities.
- All garbage is collected and either recycled or discarded as hazardous waste. An inadvertent discharge of tungsten carbide powder during transportation is a conceivable exposure scenario for the environment, however no such instances have been documented to far.
Frequently Asked Questions (FAQ)
What is tungsten carbide?
Tungsten carbide is a tungsten-carbon alloy formed by heating tungsten powder with carbon and hydrogen at temperatures ranging from 1,400 to 1,600°C (2,550 to 2,900°F). The resultant alloy is 2-3 times more rigid than steel and has compressive strength that exceeds all known melted, cast, and forged metals. It has a high degree of deformation resistance and maintains its stability in conditions that might be extremely hot or cold.
How strong is tungsten carbide?
Tungsten carbide is exceptionally hard, having a Mohs hardness of 9 to 9.5 and a Vickers number of roughly 2600. In comparison, the Mohs hardness of diamond is 10, with diamond having the greatest hardness standard.
While tungsten carbide is not as hard as diamond, it has other physical properties that are far superior than diamond. Its rigidity, for example, can be twice that of steel, with a Young’s modulus of around 530-700 GPa, which is likewise twice that of steel.
Is tungsten carbide magnetic?
Tungsten carbide is an alloy whose magnetic properties are determined by either the Cobalt or Nickel binder. Furthermore, cobalt significantly attracts a magnet, but nickel does not. Tungsten Carbide will not attract a magnet if the binder is nickel, but it will if the binder is cobalt.
Video on Tungsten Carbide
References
- https://www.dymetalloys.co.uk/what-is-tungsten-carbide/
- https://www.britannica.com/science/inorganic-compound
- https://www.protoolreviews.com/what-is-tungsten-carbide-tool-accessories/
- https://nanopartikel.info/en/knowledge/materials/tungsten-carbide/
- https://carbideprocessors.com/pages/carbide-parts/tungsten-carbide-properties.html
- https://blog.thepipingmart.com/metals/c2-tungsten-carbide-physical-and-chemical-properties/

