The study into the vapor pressures of mixtures comprising fully miscible liquids has proven to be highly beneficial in facilitating the separation of these liquids through the process of fractional distillation. The vapor pressures of two liquids were measured at a constant temperature, while their compositions were varied. Vapor pressure versus composition plots have shown that there are three distinct classes of combinations involving miscible liquids.
Interesting Science Videos
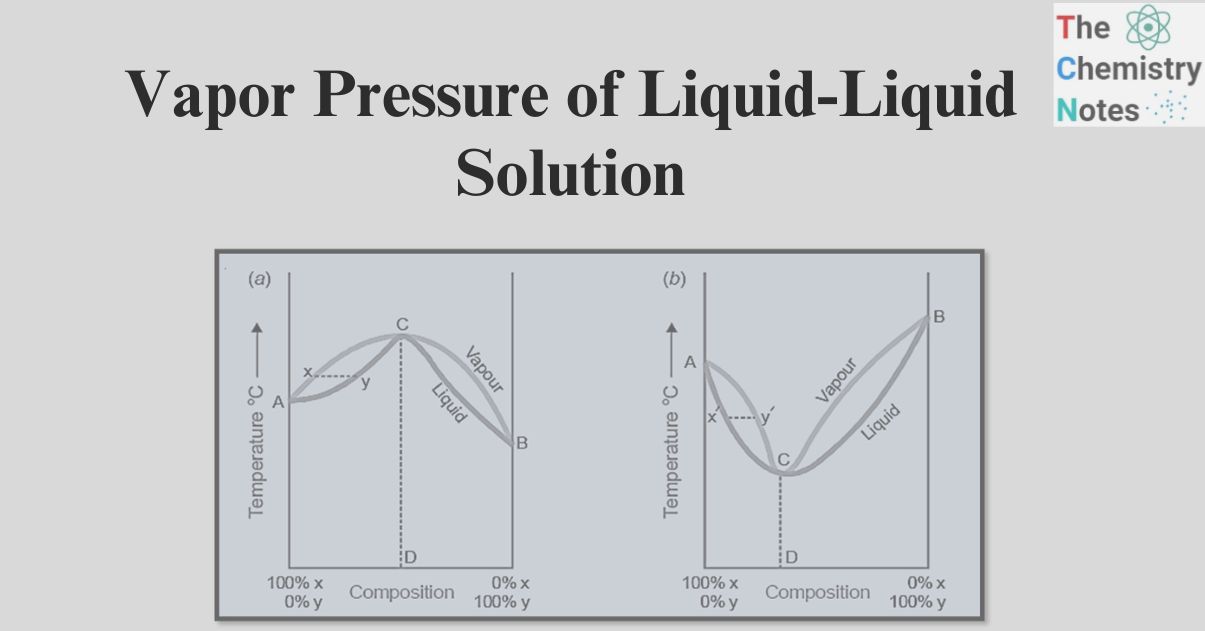
The first type of liquid-liquid solution
- In the case of these particular solutions, the vapor pressure curve demonstrates a point of minimum value.
- When considering a mixture with an abundance of component X, which is characterized by higher volatility, the point of interest lies at position C along the curve.
- Upon distillation, the resulting vapor will possess an abundance of X, consequently leading to an increased concentration of Y in the remaining mixture.
- Ultimately, we arrive at point D, where the vapor pressure is at its minimum, resulting in the highest possible boiling point.
- In this process, the mixture will undergo distillation without any alteration in its composition.
- In a similar manner, when a mixture with a higher proportion of Y (represented by point E) is subjected to distillation, Y will vaporize and leave behind a residue that is enriched in X.
- This process continues until the mixture reaches the minimum point D, at which the distillation results in no change to the composition of the mixture. It is evident that achieving a complete separation of this category of solutions into distinct components is unattainable.
In the most optimal case, the mixture can be separated into a single, pure component and a constant boiling mixture. Solutions that exhibit a maximum boiling point and remain unchanged at a constant temperature are referred to as maximum boiling point azeotropic solutions. One prominent illustration of this particular category is exemplified by hydrochloric acid, which establishes a consistent boiling mixture at a temperature of 110°C and consists of 20.24% acid content. When distilling a mixture of any composition, either hydrochloric acid or water will vaporize and pass over. The composition of the mixture will move to the point of minimum vapor pressure during distillation, without undergoing any changes in its composition.
Second Type of liquid-liquid solution
- The vapor pressure curve exhibits a peak at point F.
- At this juncture, the mixture exhibits the utmost vapor pressure, consequently resulting in the most minimal boiling point.
- Therefore, in this particular type of solution, the initial fraction will comprise a constant boiling mixture with a predetermined composition that corresponds to the maximum point, until the complete depletion of one component occurs.
- Subsequently, there will be an increase in temperature, and the remaining component will undergo vaporization.
- In this particular type of solution, achieving a complete separation through fractional distillation is not feasible. In the optimal scenario, we can separate it into a mixture that boils at a constant temperature and one component that exists in a pure state.
- Ethanol and water mixtures serve as a notable illustration of this phenomenon.
- The boiling point of an ethanol-water mixture with a concentration of 95.6% ethanol is observed to be 78.13°C, which represents the lowest temperature at which the mixture undergoes vaporization.
Obtaining pure absolute alcohol through distillation is a highly challenging task. However, this challenge has been successfully addressed by introducing benzene, which forms a low boiling mixture with water. Through the process of distillation, this mixture can be separated, resulting in the pure ethanol being left behind.
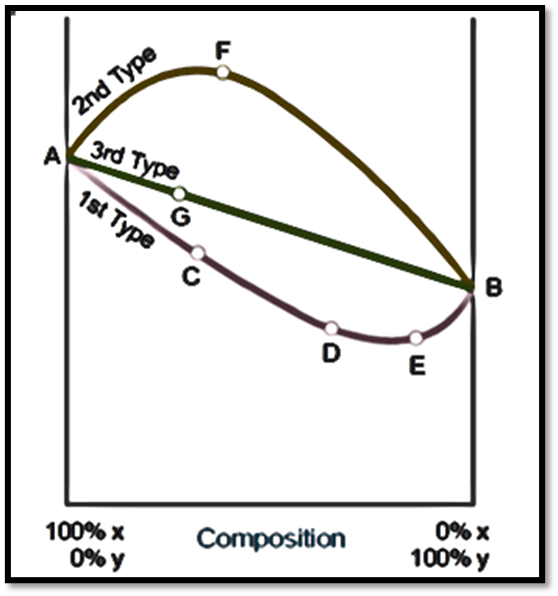
Third Type of liquid-liquid solution
- In this particular case, it can be observed that the vapor pressures of mixtures consistently fall within the range of vapor pressures exhibited by individual components.
- Consequently, the composition curve of vapor pressure demonstrates a linear trajectory. Assuming an abundance of Y within the mixture, denoted by point G on the curve.
- During the process of distillation, component X, which exhibits higher volatility, will be obtained in a larger proportion in the distillate.
- This phenomenon can be observed as we progressively move along the curve AB.
- The subsequent fractions will exhibit a decrease in X content and an increase in Y content until the point of reaching 100% on the Y-axis, at which all X will have been transferred.
- By iteratively employing the process of distillation using the newly obtained distillate, which exhibits an increased concentration of component X, it is possible to obtain a nearly pure form of component X.
Fractional distillation is the sole method by which the components can be fully separated in this particular type of solutions. Therefore, the separation of methyl alcohol-water mixtures into their respective pure components can be achieved through the process of distillation. Zeotropic mixtures are liquid mixtures that undergo distillation with a discernible alteration in their composition.
Fractional distillation
The previous section of our discussion focused on the vapor pressure composition curves pertaining to the three distinct types of solutions. From our analysis, we can deduce that only the third type of solution allows for a thorough separation through the process of distillation.
What is fractional distillation?
Fractional distillation is a distillation technique employed for the purpose of separating miscible liquids. The fractional distillation process encompasses a series of iterative condensation and distillation steps. Fractional distillation is typically employed for the purpose of separating the constituent elements of a mixture. The process of separation occurs when the mixture is subjected to heat at a specific temperature, causing certain fractions of the mixture to undergo vaporization. The fundamental principle underlying fractional distillation is the differential volatility of liquids, which causes them to evaporate and boil at distinct temperatures. Hence, in the process of fractional distillation, when the mixture is heated, the component with a lower boiling point will undergo vaporization prior to the other components, resulting in its conversion into vapor.
Fractional distillation process
In order to comprehend the process of fractional distillation, it is imperative to possess a comprehension of the composition of both the vapor phase and the liquid mixtures at varying boiling temperatures. Therefore, in order to achieve this objective, the temperature-composition curve holds greater significance compared to the vapor-pressure composition curve. When the boiling point of a liquid mixture is graphed against its composition and the composition of the vapor in contact with it, distinct curves are observed for each type of solution.
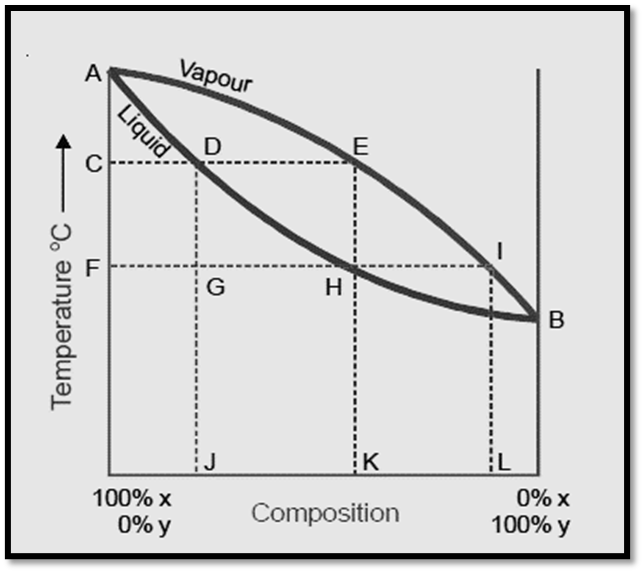
- The temperature composition curves for the vapor and liquid are denoted as AEB and ADB, respectively.
- At any given boiling temperature, denoted as C, the composition of the liquid mixture is symbolized as J, while the composition of the vapor in equilibrium is represented by K.
- Evidently, the volatile component Y is found in a higher proportion in the vapor phase compared to the liquid mixture. Consequently, the condensed vapor or distillate will exhibit a higher concentration of X.
- If the distillate obtained is subsequently subjected to distillation, it will exhibit a boiling point at temperature F, and the resulting fresh distillate will possess the composition L, which corresponds to the initial substance I.
- Therefore, the relative amount of Y present in the second distillate is higher compared to the first distillate. By iteratively employing the method of fractional distillation, it becomes evident that a nearly pure form of Y can be obtained.
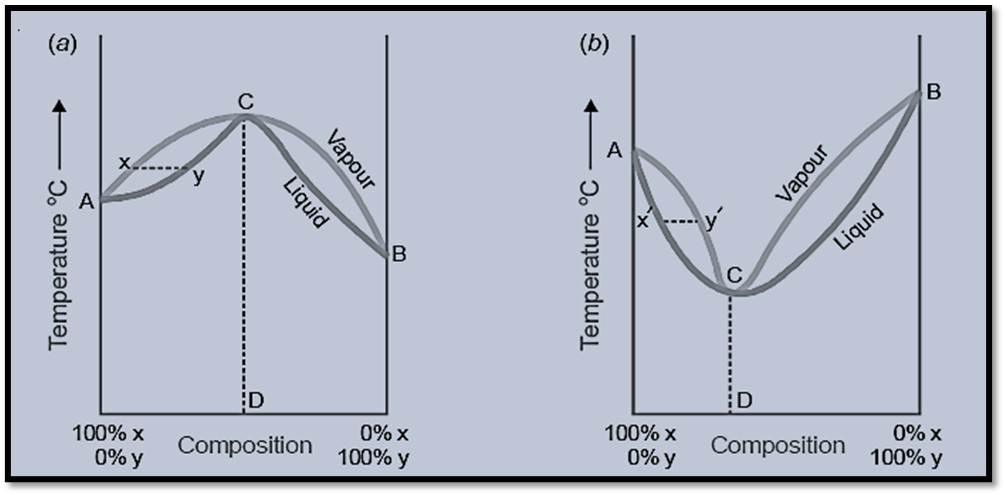
In the first type of solution (Figure a), when a boiling mixture represented by Y is present, the vapor phase will contain a lower concentration of Y compared to the liquid phase. Consequently, the boiling point will increase gradually until it reaches the maximum point C, where the composition of the liquid and vapor phases becomes identical. In this process, distillation occurs without any alteration in the chemical composition. In the same way, in the second case (as depicted in Figure Figure b), when considering a boiling mixture denoted by the point X’, the quantity of Y present in the vapor phase is greater, leading to a gradual decrease in the boiling point until it reaches the minimum point C’. At this minimum point, the compositions of the vapor and liquid mixtures are identical. At the given temperature, the mixture undergoes boiling while maintaining its original composition. Therefore, it has been demonstrated that fractional distillation is unable to separate the second and first types of solutions.
The utilization of what is known as fractionating columns contributes to a sizeable improvement in the overall productivity of the process of fractional distillation. These come in a variety of designs. A long glass tube that is packed with glass beads (Fig. a) or specifically produced porcelain rings is the component that makes up an efficient and straightforward fractionating column that is typically used in laboratory settings. A fractionating column might also be made out of the glass tube that has been blown into bulbs at regular intervals. A fractionating tower, illustrated in figure b, is utilized for industrial reasons.
Using trays that are stacked on top of one another, a fractionating tower can be subdivided into a number of different sections. Each tray has a bubble cap covering a hole in the middle of it. These holes are covered by bubble caps. Each tray is equipped with an overflow pipe that connects it to the tray below it and enables the liquid that has been condensed to flow down.
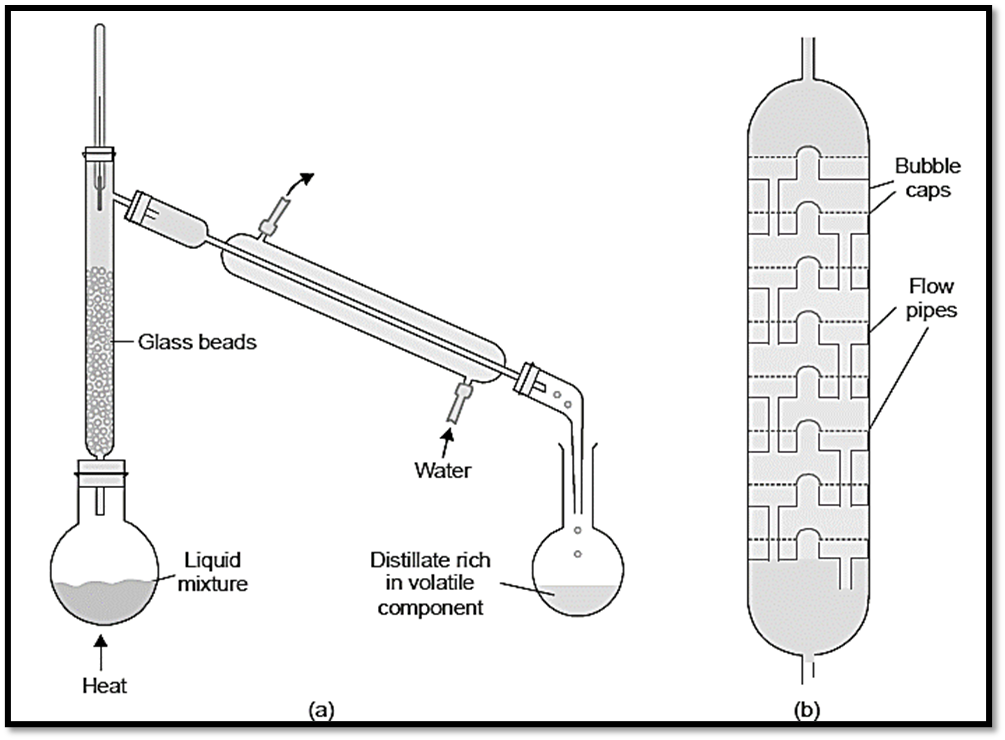
- The fractionating column, also known as a tower, is installed within the neck of the distillation flask or still, allowing the vapors of the heated liquid to ascend through it.
- The temperature decreases within the column as vapors ascend from the lower section to the upper section.
- The thermal vapors that are introduced into the column undergo initial condensation in the lowermost section of the column.
- As the process of heating persists, an increased number of vapors ascend the column and cause the previously condensed liquid to boil.
- Consequently, a vapor is formed which condenses at a higher point within the column.
- The liquid undergoes heating as a result of the subsequent ascent of additional vapors within the column.
- Consequently, the liquid that undergoes condensation in the lower section is subsequently distilled into the upper section.
- In this process, there is a phenomenon of distillation and condensation occurring throughout the vertical extent of the column.
- This leads to an elevation in the concentration of the volatile constituent within the exiting vapors.
- At each point within the column, a state of equilibrium is established between the liquid and vapor phases.
- The establishment of this phenomenon is facilitated by the upward movement of vapors and the downward movement of liquid, accompanied by a significant surface area and a gradual distillation rate.
Fractional distillation of crude oil
One prevalent application of fractional distillation in industrial settings involves the segregation of different constituents found within crude oil. Typically, crude oil comprises various components, including paraffin wax, gasoline, diesel, naphtha, lubricating oil, and kerosene. The distillation process facilitates the efficient separation of these components.
The chamber is filled with crude oil, which is subsequently subjected to elevated temperatures through the application of high-pressure steam. The mixture undergoes a phase transition, resulting in the formation of vapor as it reaches its boiling point. At this juncture, a multitude of substances undergoes a phase transition into the gaseous state. The vapor ascends within the fractional distillation column, which is comprised of multiple plates. The plates possess apertures that facilitate the passage of vapor. Typically, a low temperature is maintained at the uppermost section of the fractionating column. In this context, it can be observed that components possessing higher boiling points will undergo condensation in the lower section of the column, whereas substances characterized by lower boiling points will experience condensation at the uppermost region. The vapor or liquid fractions that have been condensed are subsequently extracted from the lateral sections of the column. The liquid fractions that have been collected can be subjected to further cooling by passing them through condensers.
Fractional distillation of ethanol
Despite the distinct boiling points exhibited by ethanol and water, fractional distillation is unable to achieve complete separation of these components within a mixture. The boiling point of water is observed to occur at 100 degrees Celsius, whereas ethanol exhibits boiling at a lower temperature of 78.4 degrees Celsius. When subjecting an alcohol-water mixture to boiling, it is observed that the ethanol content in the vapor increases. However, this phenomenon is limited due to the formation of an azeotrope between alcohol and water. Upon reaching a composition of 96% ethanol and 4% water, the mixture exhibits increased volatility, characterized by a lower boiling point of 78.2 degrees Celsius compared to that of pure ethanol.
Application of fractional distillation
- The utilization of fractional distillation proves to be advantageous in the manufacturing process of high-purity silicon derived from chlorosilanes. Silicon is widely utilized in the field of semiconductors.
- This technique is employed for the purpose of separating liquefied air. Components such as liquid nitrogen, concentrated argon, and oxygen are acquired.
- Fractional distillation is employed in diverse industries, such as oil refineries and chemical plants, primarily for the purpose of separating and purifying numerous organic compounds.
- The utilization of this method proves to be advantageous in the water purification process.
- Fractional isolation is a commonly employed technique for the separation of acetone and water.
References
- https://www.toppr.com/guides/chemistry/is-matter-around-us-pure/fractional-distillation/
- https://www.thoughtco.com/definition-of-fractional-distillation-604421
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_Lab_Techniques_(Nichols)/05%3A_Distillation/5.03%3A_Fractional_Distillation
- https://byjus.com/chemistry/fractional-distillation/
- https://en.wikipedia.org/wiki/Fractional_distillation
- https://energyeducation.ca/encyclopedia/Fractional_distillation#:~:text=Fractional%20distillation%20is%20the%20process,weights%20in%20a%20distillation%20tower.
