In an open vessel, a liquid evaporates as soon as it is placed there. Different kinetic energy is being used by the liquid’s molecules as they move. The intermolecular interactions that keep the molecules in the liquid can be overcome by those with kinetic energies that are above average. These kinetic molecules evaporate as vapour from the liquid surface. Vaporization or evaporation is the process through which liquid molecules transform into gases (vapours). Moreover, condensation is the process in which liquid molecules change from being in a gas to being in a liquid state.
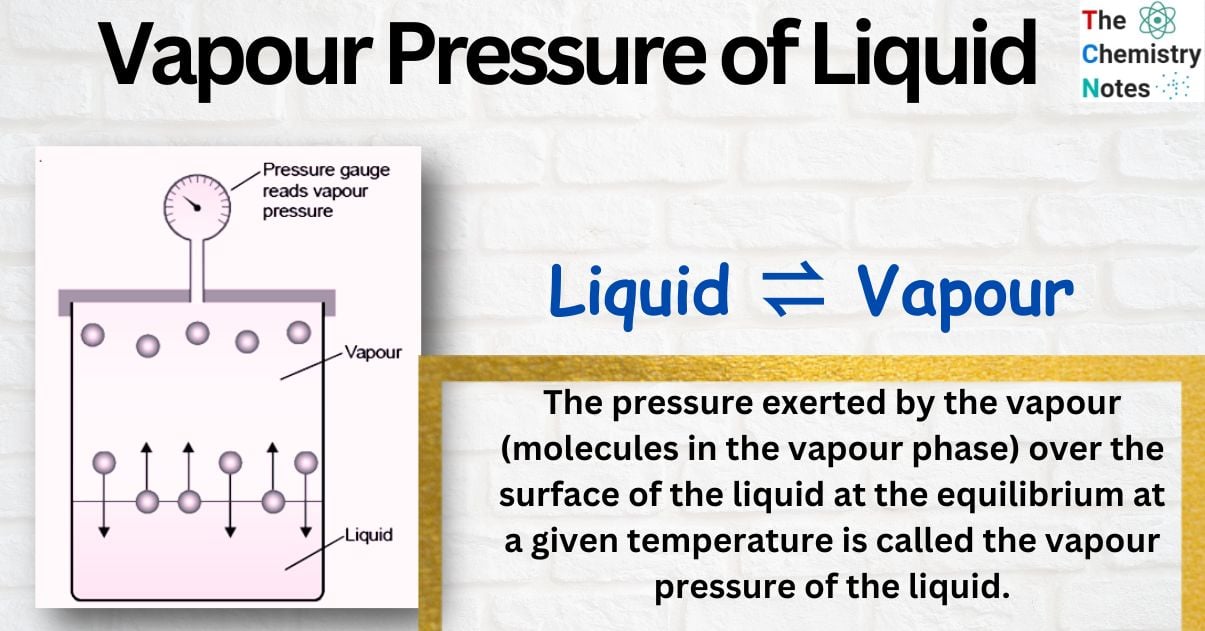
The molecules with high kinetic energy escape into space above the liquid if the liquid is placed in a closed vessel. Many molecules strike the liquid surface as the number of molecules in the gas phase rises and are trapped (condensation). When the quantity of molecules escaping from the liquid equals the quantity of molecules returning to the liquid, a phase has been reached where the rate of condensation and evaporation is exactly equal. As a result, at the specified temperature, a dynamic equilibrium is formed between the liquid and the vapour.
Liquid ⇌ Vapour
As time passes, the concentration of the vapour in the area above the liquid won’t vary. As a result, at equilibrium, the vapour will exert a specific pressure. The pressure that a liquid’s vapour exerts in equilibrium with the liquid at a constant temperature is known as the vapour pressure of the liquid. The vapour pressures of various liquids differ considerably, depending upon the identity of the liquid with its particular intermolecular forces.
Interesting Science Videos
What is vapour pressure?
The pressure exerted by the vapour (molecules in the vapour phase) over the surface of the liquid at the equilibrium at a given temperature is called the vapour pressure of the liquid.
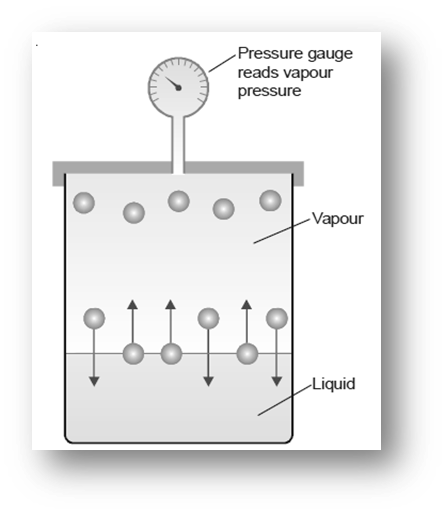
The energy of the molecules increases when the liquid is heated, making them lighter and occupying the liquid’s surface. When a liquid is heated, the molecules’ energy rises, they become lighter, and they take up more space on the liquid’s surface. This process is usually referred to as “evaporation.” The molecules that are present on the liquid surface are referred to as “vapor.” At a specific temperature, this pressure develops in a closed container during a thermodynamic equilibrium state. The equilibrium vapor pressure can be used to calculate a rate of evaporation of liquid. The temperature rises with an increase in vapour pressure. When the pressure from the environment equals the pressure from the vapor that is when the liquid reaches its boiling point.
Characteristics of Vapour Pressure
- As comparison to a liquid’s solution, a pure liquid experiences more vapour pressure.
- It has an inverse relation to the forces of attraction between liquid molecules.
- Temperature is the only factor that affects vapor pressures.
- As the temperature rises, it starts increasing. This occurs as a result of the molecules’ increased kinetic energy and rapid vaporization.
- The vapor pressure of a liquid is independent of the volume of the liquid in the container.
- It is significant to remember that when a liquid boils, the pressure of its vapor equals the atmospheric pressure.
Factors affecting vapour pressure
- Nature of Liquid: Intermolecular forces are used to characterize the liquid and explain its properties. That is to say, if we increase the strength of the intermolecular interactions, we will see a decrease in the vapour pressure.
- Effect of Temperature: When a liquid is heated, its kinetic energy rises concurrently with the rising temperature. The higher the kinetic energy, the greater the escape tendency of the molecule, and the higher the vapour pressure. This suggests that there is a direct relationship between temperature and the vapor pressure.
- Concentration of Solute: Vapor pressure is drastically decreased when a solute is present in a liquid. Also, the rate at which the vapour pressure drops depends on the solute concentration.
- Humidity: Vapor pressure for a given quantity of water vapor in the air is independent of any other parameter except temperature. In order for humidity to have an effect, all other factors must remain unchanged. There should be no misunderstanding about the relative importance of temperature and humidity.
- Volume: Vapor pressure is not normally impacted by changes in container volume. We all know that the liquid inside the box and the vapor above it will be in a state of balance. Now, let’s assume that we divide the volume of surface S into infinite elementary volumes, so causing a change in volume, say a decrease; this would cause some of the vapor in the container to condense into a liquid. And if the volume increases, some of the liquid will boil off, becoming a vapour.
- Surface area: Generally, vapour pressure is independent of surface area.
Unit of Vapour Pressure
The torr is the standard measurement of vapour pressure. The equivalent of one millimeter of mercury corresponds to one torr. Vapor pressures of most materials are measured from the outside. At standard temperature (22 °C = 72 °F), the vapour pressure of water is around 20 torrs. Keep in mind that water vapour pressure will be 760 torr = 1 atm when it reaches its boiling point at 100oC. (212 oF).
Determination of vapour pressure
The vapour pressure of a given liquid can be measured by Static method or Dynamic method.
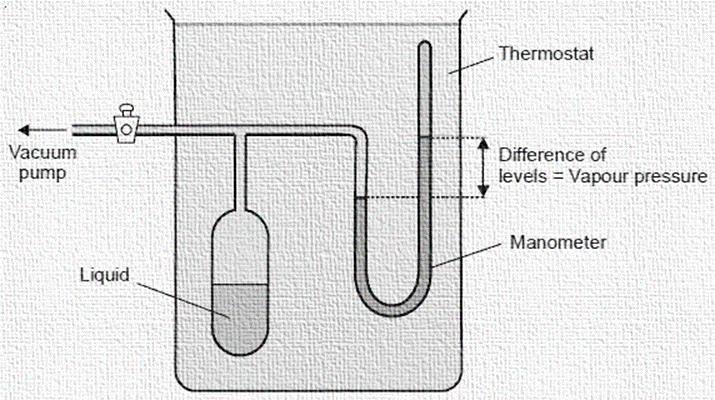
Static method
A vacuum pump and mercury manometer are connected to a bulb containing the liquid whose vapour pressure needs to be determined. Working the vacuum pump and closing the stopcock removes all of the air from the bulb. It evaporates off some of the liquid. The system is then held at the constant temperature for long enough for equilibrium to be reached. The manometer’s separating mercury levels represent the liquid’s vapor pressure. The vapour pressure of liquid at a different temperature can be calculated by adjusting the thermostat. The vapour pressure of a liquid up to one atmosphere can be handled by this technique.
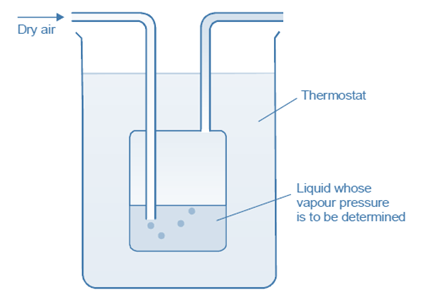
Dynamic method
The given liquid is heated in a constant flow of inert gas (T). The vaporized gas of liquid is released from the flask through the tube. If V be the volume of the gas passed and m the loss in weight of the liquid, the vapour pressure is given by the expression
Vapour pressure = (m/MV)× RT
where.
M = molecular weight of the liquid and
R = gas constant.
This method is particularly suited for liquids of very low vapour pressure.
Effect of Temperature on Vapour Pressure
The vapour pressure of a liquid will rise if its temperature is raised. This is because a greater number of molecules in the liquid will have a higher kinetic energy and break away from the liquid surface as the temperature of the liquid increases. This means that the concentration of vapour molecules will rise before returning to equilibrium. The average kinetic energy of the molecules comprising the vapour also rises as the temperature rises. Temperature is directly related to the concentration and kinetic energy of vapour. Therefore, if you raise the temperature, the vapour pressure will rise as well.
Effect of Vapour Pressure on Boiling Points
Tiny bubbles form in a liquid when it is heated. When these reach the liquid’s surface, they explode. The point at which the liquid begins to boil is known as its boiling point. Let’s think about a single bubble for a moment. The liquid evaporates into the bubble, and the vapour pressure within the bubble maintains the bubble’s shape. However, the bubble often bursts due to the atmospheric pressure acting on the liquid surface. The vapour pressure in the bubble balances out with the air pressure at the surface as the bubble rises. This causes the bubble to burst. Therefore, it is possible to define the boiling point of a liquid as the temperature at which its vapour pressure is equivalent to that of the surrounding air.
Boiling points are reported at 1 atm (760 torr) to account for variations in atmospheric pressure due to altitude and other factors . In other words, a liquid’s normal boiling point is when its vapour pressure is 760 torr, or 1 atmosphere.
A liquid’s boiling point can be lowered by using a vacuum pump to reduce the surrounding pressure. The vapour pressure of liquid would then be the same as the ambient pressure. Increasing the pressure around a liquid raises its boiling point. At higher temperatures, atmospheric pressure exactly balances the liquid’s vapour pressure. This is the principle upon which a home pressure cooker relies. Since the pressure inside the cooker is kept above 1 atmosphere, the liquid inside would boil at a temperature greater than 100 degrees Celsius. This reduces the total time needed for cooking.
Vapour Pressure of Pure Liquids
Vapor pressure is a temperature-dependent measurement of a substance’s ability to change phase from solid to gas or vapour. Specifically, the boiling point of a liquid is the temperature at which the vapour pressure at its surface is equal to the pressure of its environment. The vapour pressure of a liquid is defined as the pressure that is exerted above the liquid when it is in thermal equilibrium with the surrounding vapour.
Vapour Pressure of Liquid-Liquid Solutions
We use two different evaporable liquid solutions to illustrate this phenomenon. Let’s call the two liquid parts of their make-up A and B. When a volatile liquid and its constituents are contained in an airtight container, the liquid and vapor phases reach a state of equilibrium. Let PA and PB represent the partial vapour pressures of A and B, respectively, and let Ptotal represent the total vapour pressure at equilibrium. In addition, xA and xB represent the mole fractions of their respective components. The vapour pressure of volatile liquids is calculated using Raoult’s Law.
Raoult’s Law
Raoult’s law states that the partial pressure is directly proportional to the mole fraction of the solute component.
As per Raoult’s Law, the partial pressure of A will be
PA ∝ xA
PA = PA0 xA
where PA0 is the vapor pressure of element A in its purest liquid form. In a similar vein, partial pressure of B will be;
PB ∝ xB
PB = PB0 xB
when Component B is a pure liquid, the vapor pressure is PB0.
Dalton’s law of partial pressures will now be used further.
Dalton’s Law of Partial Pressure: According to this law, when a solution is contained within a container, the total pressure (Ptotal) of the solution is equal to the sum of the partial pressures of the solution’s individual components. As in
Ptotal = PA + PB
Ptotal = PA0 xA + PB0 xB
since, xA + xB = 1 ,
Ptotal = PA0 + (PB0 – PA0) xB
Vapour Pressure of Solid-Liquid Solutions
Now we’ll take a look at the other common type of solution, one in which solids are dissolved in a liquid. In this case, the solute is the solid form, and the solvent is the liquid. In these instances, the solute does not evaporate. When compared to the solution’s pure vapor pressure, the vapour pressure is lower.
Let’s look at how to calculate the solution’s global vapour pressure now. Let’s pretend that A is the solvent and B is the solute in a solution. Using Raoult’s Law, we can determine that the partial vapour pressure of a given component (solute/solvent) is proportional to the mole fraction of that component.
Now, when we incorporate a non-volatile solute, it is crucial that the vapour pressure originates solely from the solvent component. Because nothing else exists in the vapor state, they are crucial. According to Raoult’s law, the relationship between the vapor pressure of a solvent (PA), the mole-fraction of that solvent (xA), and the vapor pressure of pure solvent (PA0) is as follows:
PA ∝ xA
PA = PA0 xA
The relationship between the solvent mole fraction and the vapor pressure is linear.
There are other two primary formulas for vapour pressure:
- Clausius-Clapeyron
- Henry’s Law
Dühring’s Rule
Eugen Dühring discovered Dühring’s rule, which claims a linear connection exists between the temperatures at which two solutions exert the same vapour pressure. Frequently, the rule contrasts an unadulterated liquid with a solution of a specified concentration.
Dühring’s plot is a graphical representation of this relationship, with the boiling point of the purified liquid along the x-axis and the boiling point of the mélange along the y-axis; each line of the graph represents a constant concentration.
This video explains vapor pressure and boiling. Vapor pressure or vapour pressure or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system.
References
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012
- Petrucci, Ralph H., et al. General Chemistry: Principles and Modern Applications. Upper Saddle River, NJ: Prentice Hall, 2007.
- Halliday, David; Resnick, Robert; Krane, Kenneth S. (2010-04-20). Physics, Volume 2. John Wiley & Sons. p. 342.
- https://byjus.com/chemistry/liquid-state-vapour-pressure/#:~:text=A%20liquid’s%20vapour%20pressure%20is,sample%20in%20a%20closed%20container.
- “Vapour pressure of Pure Liquid Organic Compounds: Estimation by EVAPORATION”. Tropospheric Chemistry Modelling at BIRA-IASB. 11 June 2014
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/States_of_Matter/Properties_of_Liquids/Vapor_Pressure
- https://www.toppr.com/guides/chemistry/solutions/vapour-pressure-of-liquid-solutions/
- Atkins, P.W. and Julio de Paulo, Atkins’ Physical Chemistry, Oxford University Press, UK, Indian Edition 9, 2011.
- R. Chang, “Physical Chemistry for the Chemical and Biological Sciences”, University Science Books, Sausalito, California (2000).

