In chemistry, it’s important to recognize that water molecules can be found in crystalline solids. This is often referred to as water of crystallization or water of hydration.
The phenomenon of water crystallization applies to the molecular arrangement of salt in its crystalline form. Within this particular state, a predetermined quantity of water molecules is covalently bonded to the salt molecules. The inclusion of water is a crucial factor in the determination of both the morphology and pigmentation of crystals.
Water is often present when crystals form in water-based solutions. The term “water of crystallization” refers to the amount of water molecules in a substance at a certain temperature. Water molecules are usually found in a specific amount compared to the other parts of the substance. In classical discourse, “water of crystallization” refers to water molecules trapped within a metal complex or salt compound’s crystal structure. The water molecules are not directly connected to the metal cation. Proteins tend to form crystals when there is a lot of water in the crystal structure, unlike inorganic salts. Proteins usually contain about 50% water.
Interesting Science Videos
How Does Water of Crystallization Form
- By forming crystals out of an aqueous solution, many chemicals can be purified.
- Water can fit inside the crystalline lattice without being chemically bonded to the cation of the compound, despite the fact that the crystal rejects many impurities.
- This water can be removed by heating it, although doing so usually results in damage to the crystal structure. If getting a pure compound is the goal, then this is acceptable.
- When developing crystals for crystallography or other uses, it might not be desirable.
- These crystals are free from any impurities.
- Heat has a significant impact on the crystals. The water of crystallization, also known as hydration water, is composed of water molecules that are found within crystals.
- Water is frequently present during the formation of crystals from solutions.
- The term “water of crystallization” pertains to the quantity of water present in a substance at a particular temperature.
- Under different conditions, it usually maintains a consistent ratio.
- The phrase “water of crystallization” applies to water found within the crystal structure of a metal complex or a salt, but is not directly bonded to the metal cation.
Nomenclature of Water of Crystallization
In molecular formulas, the presence of water of crystallization is sometimes indicated in different ways, but the information provided can be unclear or not specific enough. The definitions of the terms “hydrated compound” and “hydrate” are often not very clear.
- There are two ways to represent water of crystallization in molecular formulas.
- Hydrated compound·nH2O For example, CaCl2·2H2O
- Hydrated compound(H2O)n For example, ZnCl2(H2O)4
Sometimes, the two forms can be put together.
One way to describe the water of crystallization of copper(II) sulfate is by using the formula [Cu(H2O)4]SO4·H2O.
Other Solvents In Crystals
- Water is a small molecule having polar properties. It is easily incorporated into crystal formations. It is crucial to note, however, that other solvents can also be found in crystals.
- In fact, the vast majority of solvents remain within the crystal to some extent. Benzene is a common example used to demonstrate this principle.
- Chemists utilize vacuum extraction and heat to extract as much solvent as possible while minimizing its impact on a sample.
- X-ray crystallography is frequently used to detect solvent within a crystal.
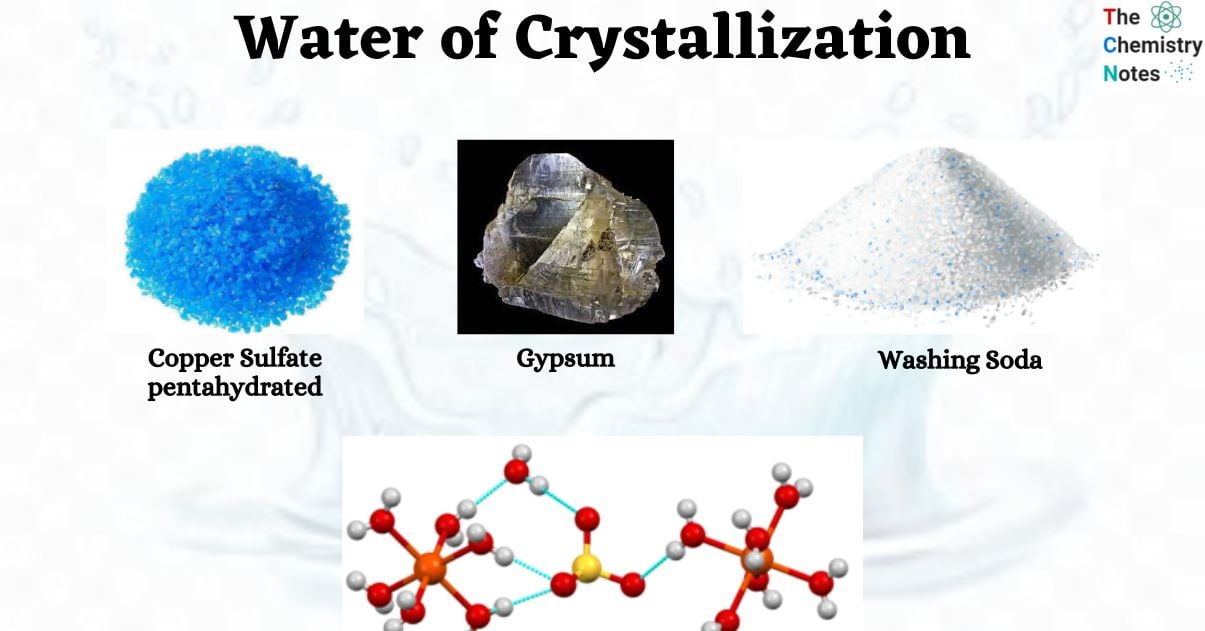
Common Examples of Water of Crystallization
- Washing soda (Na2CO3.10H2O) This compound is classified as an inorganic hydrate of sodium carbonate and exhibits a visually pleasing white or colorless crystalline salt-like structure.
- Copper Sulfate Pentahydrate (CuSO4.5H2O) It is a compound that consists of copper (2+) sulfate and water molecules. It is known for its vibrant blue color and forms a solid structure with distinct crystals.
- Gypsum (CaSO4.2H2O) It is a mineral of the sulfate variety. It is characterized by its softness and composition of calcium sulfate dihydrate. The object exhibits a somber, sturdy, stone-like exterior.
- Tin (II) chloride dihydrate (SnCl2.2H2O) This is a white crystalline solid that can be found in our school’s chemistry laboratory.
Application of Water of Crystallization
- Understanding hydration is crucial when determining the masses of various compounds. Many salt-like solids can react differently when water is present.
- The process of hydrating and dehydrating salts plays a crucial role in the application of phase-change materials for energy storage.
Hydrated Salts
A hydrated salt refers to a type of salt molecule that forms a crystal structure and is somewhat loosely associated with a few water molecules.
- When the negative ion of an acid and the positive ion of a base combine, they create a molecule that is both an acid and a base. This process also results in the formation of salt.
- An anhydrate is a type of salt molecule that does not have any water molecules attached to it. On the other hand, a hydrated salt is a salt molecule that is connected to water molecules.
- In a hydrated salt, the water molecules are incorporated into the crystalline structure of the salt. A hydrated salt is a specific kind of salt that contains water molecules connected to its ions within its crystalline structure.
- The water molecules that were mentioned are often referred to as crystallization fluids or hydration waters, which is something that is widely known.
- Hydrated salts are a specific category of salts that contain water molecules within their crystal structure.
- In every formula unit of a hydrated salt, there exists a fixed and constant number of water molecules that are involved in the process of crystallization.
Action of Heat on Hydrated Salts
When hydrated salts are exposed to elevated temperatures, they experience a phenomenon called dehydration. During this process, the water molecules that are incorporated into their crystal structure are removed.
- When the water of crystallization is lost, the hydrated salts experience a transformation in both their physical form and appearance.
- The original form and appearance of the objects are no longer maintained, as they undergo a transformation into particles that lack color and have a texture resembling powder.
- When anhydrous salts lack water of crystallization, they experience hydration and return to their initial color upon the addition of water.
For Example,
When heated
Δ
CuSO4.5H2O → CuSO4 + 5 H2O
(Hydrated copper (Anhydrous Water
sulfate) copper sulfate)- The color of the copper sulfate crystals is blue. When copper sulfate crystals are exposed to elevated temperatures, they experience a phenomenon known as dehydration, which leads to the elimination of all water molecules.
- The crystals undergo a transformation and become anhydrous copper sulfate, which is white in color.
Applications of Hydrated Salts
There are different use for hydrated salts some of which are listed here:
- Hydrated salt is utilized for water-softening industrial as well as home water-softening systems as well. Additionally, the utilization of hydrated salt is commonly observed in the alternative energy sector because it has the capacity to maintain a steady temperature for an extended period of time.
- Salt, along with hydrated salts, is utilized for a multitude of industrial applications. Hydrated salt is used by many industries. In the chemical industry, it is crucial to acknowledge that salt is the primary ingredient in more than 50% of the products. Hydrated salt finds applications in different industries like glass, paper, rubber, and textiles.
- One popular form of hydrated salt is known as Epsom salts. Essential nutrients for human health can be found in salts. It’s possible that digestion alone won’t be enough to absorb these nutrients. People have been taking therapeutic baths in areas rich in naturally occurring hydrated salts for ages. It is believed that these baths aid in healing due to their therapeutic characteristics. Epsom salts are an extremely versatile form of table salt. Although Epsom salt is often used as a home treatment, there is little evidence to support its efficacy. There are numerous commercial uses for hydrated salt.
Common Examples of Hydrated Salts
Hydrated salts are present in various states, one of which is freshwater. Salt possesses a highly adaptable crystalline structure, which enables it to readily form bonds with water molecules and undergo hydration.
- Sodium Chloride (NaCl), also known as salt, has the ability to absorb water vapor from the air or when it comes into contact with liquid water.
- When certain compounds in the soil or rock of a specific area dissolve and mix with groundwater, they form salt molecules.
- These chemicals freely flow and eventually become hydrated with water molecules.
The following are the common examples of hydrated salt:
- Sodium Carbonate (Na2CO3) crystals, which are commonly referred to as washing soda crystals, possess the chemical formula Na2CO3.10H2O. The notation is used because each unit of the formula contains 10 molecules of water of crystallization. The compound is commonly referred to as sodium carbonate decahydrate.
- Copper sulfate crystals, which are also referred to as CuSO4.5H2O, are composed of five molecules of water of crystallization in every formula unit. The chemical name for it is known as copper sulfate pentahydrate.
- The chemical formula for calcium sulfate crystals, which are also referred to as gypsum crystals, is CaSO4.2H2O. The reason behind this is that each formula unit is composed of two molecules of water of crystallization. Another term for it is called calcium sulfate dihydrate.
Frequently Asked Questions (FAQs)
Why do salts that have water in them during crystallization seem to be completely dry?
The water that forms crystals is a part of how water is made up. Since the water used to make crystals isn’t free water, it doesn’t wet the salt. So, the salts that have water of crystallization look like they are completely dry.
How does the water of crystallization benefit the salt crystals?
The presence of crystallization water influences the shape and color of salt crystals. Iron sulfate crystals exhibit a green coloration due to the presence of crystallization water.
References
- https://pubs.acs.org/doi/abs/10.1021/ic025915o
- https://iupac.org/wp-content/uploads/2016/07/Red_Book_2005.pdf
- https://scripts.iucr.org/cgi-bin/paper?S1600536802002192
- https://www.chemeurope.com/en/encyclopedia/Water_of_crystallization.html
- https://www.geeksforgeeks.org/water-of-crystallization/
- https://www.bbc.co.uk/bitesize/guides/zrx32sg/revision/10
- https://www.freechemistryonline.com/water-of-crystallization.html

