Abbreviated as SHE, the Standard Hydrogen Electrodes, a redox electrode, is the foundational basis of the scale of the oxidation-reduction potential. Moreover, it is the main electrode where the electrochemical reaction takes place. It consists of a platinum electrode immersed in a solution of 1 M HCl and surrounded by a gaseous atmosphere of hydrogen at a pressure of 1 bar.
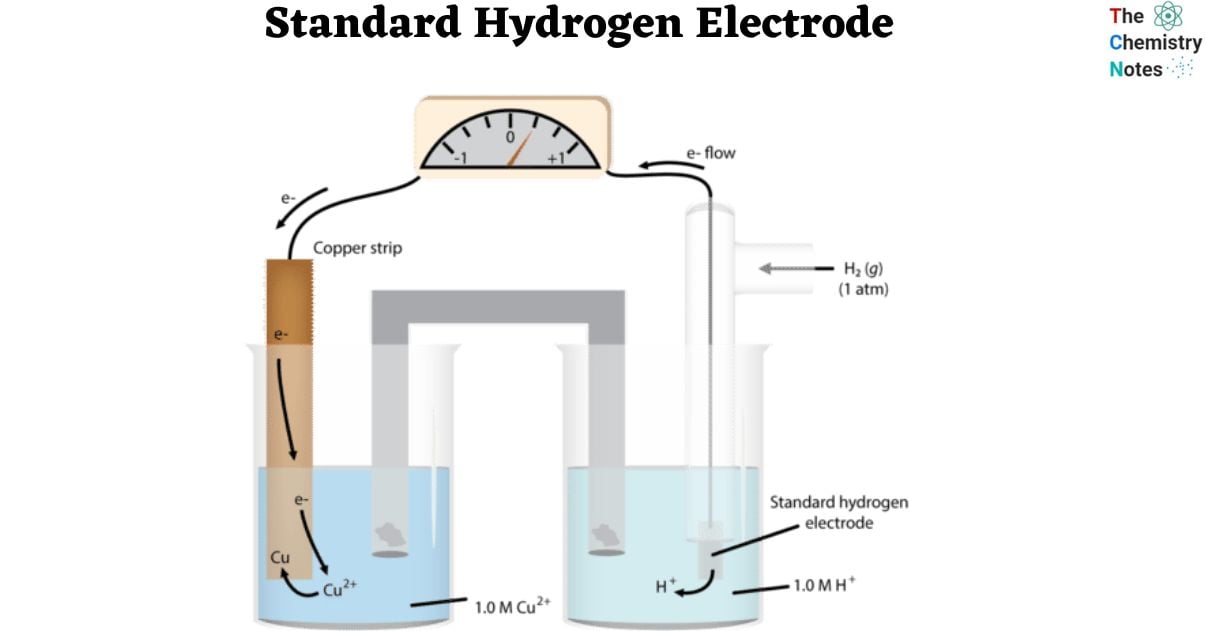
To compare hydrogen’s potential with other electrochemical reactions, it is said to have a zero volts potential (E°) at a certain temperature (298 K). At a given temperature, the potential of any electrode is compared to the potential of a hydrogen electrode. It is referred to as the standard hydrogen electrode because it acts as a reference electrode.
Platinum is used in the standard hydrogen electrode for the following reasons.
- Platinum is an inert catalyst. So, it doesn’t corrode during the electrochemical and speeds up the rate of the reaction.
- Platinum is a less reactive metal. So, it doesn’t easily react with other metals. As a result, it provides the surface for the redox reaction.
- Being a good absorber of hydrogen, platinum improves the chemical kinetics of the electrochemical reaction at the interface.
- Unlike other metals such as gold, silver, copper, mercury; platinum doesn’t poison the electrode of another half-cell.
- Owing to its catalytic properties, platinum promotes the proton reduction reaction.
Interesting Science Videos
Construction of Standard Hydrogen Electrode
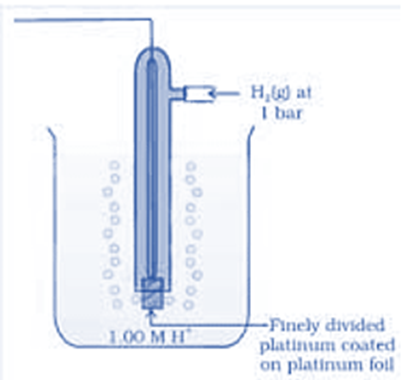
The following equipment is required for the construction of the standard hydrogen electrode.
- Hydrogen Gas/Blow
- Platinized platinum electrode
- Acid solution with molarity equal to 1 mol dm−3 (activity H+ = 1 mol dm−3)
- Reservoir to attach the second half-element of the galvanic cell
Working of a Standard Hydrogen Electrode
- The half-cell where the process of oxidation in a Galvanic cell occurs is called anode. Anodes have a negative potential relative to the solution.
- The other half-cell in a Galvanic cell where reduction takes place is called cathode. Cathode has a positive potential.
- A potential difference between these two electrodes, i.e., cathode and anode is created.
- When the switch is in the on position, the electrons start flowing from one end (negative electrode) to another end (positive electrode) in the cell.
- In the Galvanic cell, the current flows from the opposite direction to that of the electron flow.
- Cell potential is the potential difference between the two electrodes (and anode) of a galvanic cell. The unit of cell potential is volts.
- When no current is drawn through the cell, the force applied is called the cell electromotive force.
- As per IUPAC convention, the anode is on the left and the cathode is on the right in the galvanic cell.
Cells are considered positive if their emf is positive. The formula for emf is given by the potential of the half-cell (right hand side) subtracted with the potential of the half-cell (left hand side).
E (cell) = E (right) – E (left)
The cell reaction can be understood with the help of the following example:
Cu(s) + 2Ag+(aq) → Cu2+(aq) + 2 Ag(s)
Half-cell reactions is as follows:
Cathode (reduction):
2Ag+(aq) + 2e– → 2Ag(s)
Anode (oxidation):
Cu(s) → Cu2+(aq) + 2e–
Advantages of Standard Hydrogen Electrode
- Due to minute potential, the potential of a standard hydrogen electrode is taken as zero, therefore, acts as reference point for other metals.
- Standard Hydrogen Electrode performs the dual function as a cathode half-cell as well as anode half-cell.
- It can be used to determine the unknown potential of the half cell.
Disadvantages of Standard Hydrogen Electrode
- Difficulty in transportation, construction, and maintenance.
- Difficulty in maintaining the pressure of hydrogen gas.
- Difficulty in levelling the concentration of the acid solution.
- Difficulty in obtaining pure hydrogen gas.
- Difficulty in the construction of an ideal platinum electrode.
- Impurities reduce the life cycle of platinum electrode which in turn increases the costing.
- Platinum is expensive.
Calomel Electrode
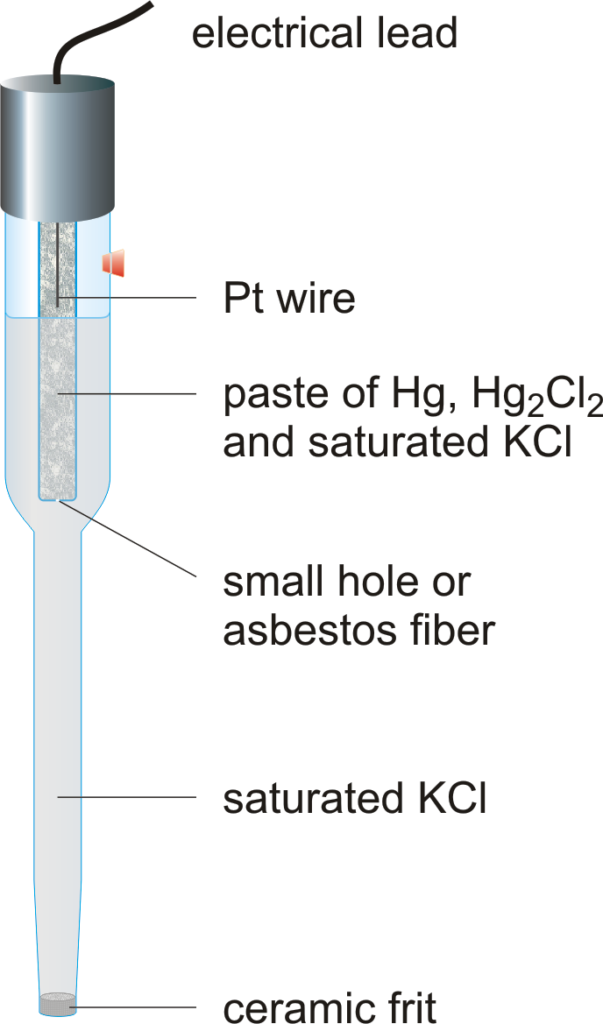
[Source: Chemistry glossary]
- The calomel electrode is the mercury-mercurous chloride electrode.
- It consists of a glass vessel having a bent side tube.
- Pure mercury is placed at the bottom of the tube, which is covered with a paste of mercury- mercurous chloride (Hg+Hg2Cl2) that is calomel.
- The remaining portion of the cell is filled with a solution of normal (1 N) or saturated KCl (Potassium chloride).
- A platinum wire sealed into a glass tube is dipped into a mercury layer and is used to provide external electrical contact.
- The side tube is used for making electrical contact with a salt bridge.
- The calomel electrode can act as an anode or cathode depending on the nature of another electrode of the cell.
Reaction at the anode:

Overall reaction:

Reaction at the cathode:
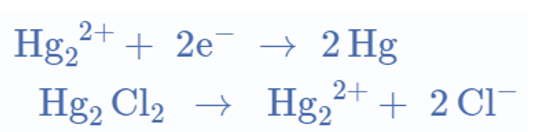
Overall reaction:

Video on Standard Hydrogen Electrode
References
- https://byjus.com/chemistry/standard-hydrogen-electrode/
- https://glossary.periodni.com/dictionary.php?en=calomel+electrode
- https://chemistnotes.com/physical/standard-hydrogen-electrodeshe-definition-diagram/
- https://unacademy.com/content/jee/study-material/chemistry/standard-hydrogen-electrode/
- https://www.sciencedirect.com/topics/chemistry/electrochemical-cell

