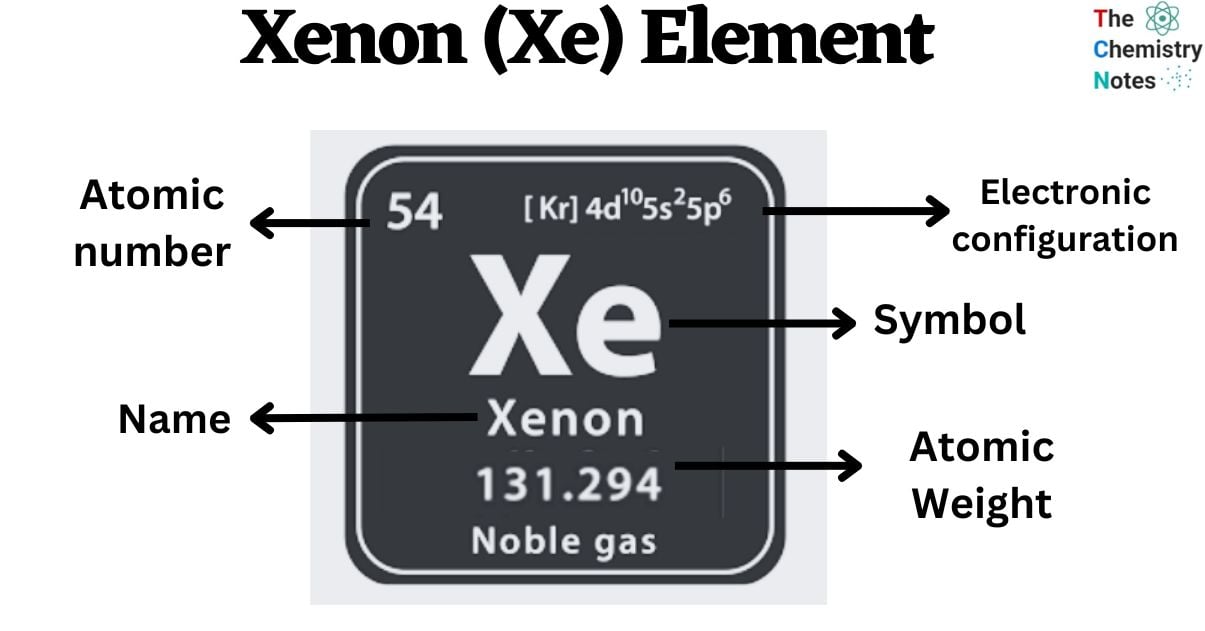
Xenon is a chemical element with the atomic number 54 and is represented by the symbol ‘Xe’ in the periodic table. It is a noble gas and belongs to the p-block of group 18 of the periodic table. It is a colorless, odorless gas that is found in trace amounts in Earth’s atmosphere. The atmosphere of Earth is made up of approximately 0.0000087% of Xe gas.
Naturally occurring xenon is made of nine stable isotopes. Xe is commercially obtained as a by-product of the separation of gases nitrogen and oxygen from the air. Due to its rarity, Xe is more expensive than other lighter noble gases.
Interesting Science Videos
History of Xenon
- On July 12, 1898, Sir William Ramsay, a Scottish chemist, and Morris M. Travers, an English chemist, discovered xenon, shortly after they discovered krypton and neon.
- Xenon was discovered the same way krypton and neon were discovered that is by employing liquefaction of the air.
- William Ramsay received the noble prize in 1904 for the discovery and co-discovery of noble gases.
- The name for the element derives from the Greek word “xenos” meaning stranger.
Occurrence of Xenon
- It is present in the atmosphere of the Earth in extremely minute quantities, with an average concentration of around 0.086 parts per million by volume.
- At the earth’s surface, it can be harvested from certain mineral springs that release gases.
- Liquid air, which is regular air that has been liquefied through compressing and refrigerated to extremely low temperatures, is the source from which it can be extracted for commercial use.
Isotopes of Xenon
Xenon has a total of 9 naturally occurring stable isotopes: 124Xe, 126Xe, 128Xe, 129Xe, 130Xe, 131Xe, 132Xe, 134Xe, and 136Xe.
Naturally Occurring Isotopes of Xenon
| Isotopes | Natural Abundance (atom %) |
|---|---|
| 124Xe | 0.09 (1) |
| 126Xe | 0.09 (1) |
| 128Xe | 1.92 (3) |
| 129Xe | 26.44 (24) |
| 130Xe | 4.08 (2) |
| 131Xe | 21.18 (3) |
| 132Xe | 26.89 (6) |
| 134Xe | 10.44 (10) |
| 136Xe | 8.87 (16) |
Elemental Properties of Xenon
| Electronic Configuration | [Kr] 4d10 5s2 5p6 |
| Atomic Number | 0.076 nm (+2); 0.064 nm (+3) |
| Atomic Weight | 131.29 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 18, 5, p-block |
| Density | 5.9*10-3 g.cm -3 at 20 °C |
| Ionic radius | 0.076 nm (+2) ; 0.064 nm (+3) |
| Van der Waals radius | 0.217 nm |
| Electron shells | 2, 8, 18, 18, 8 |
| Electrons | 54 |
| Protons | 54 |
| Neutrons in most abundant isotope | 78 |
Physical Properties of Xenon

- Pure Xe gas has a density of 5.894 kilograms per cubic meter, which is approximately 4.5 times that of the atmosphere of the Earth at sea level, which is 1.217 kilograms per cubic meter.
- As a liquid, Xe can reach a maximum density of 3,100 g/mL, with the triple point being the densest possible state.
- Xe melts at 111.75 °C (169.15 °F) and boils at -108.13 °C (-162.5 °F). It’s odd to use terms like “melting point” and “boiling point” while referring to gas. So, consider the antonyms of these two words. The process of solidifying is the antithesis of melting. Turning from a gas into a liquid is the inverse process of boiling. Therefore, Xe boiling point is the temperature at which the gas becomes a liquid.
- Xe belongs to the group of elements with no electronegativity; these are often known as noble or inert gases. The eight electrons in its outer valence shell make it resistant to typical chemical processes like combustion. This results in a structure with a stable minimum energy level, in which the outside electrons are firmly bound.
- Xe is an extremely uncommon gas that is tasteless, and odorless. Additionally, it does not react with other chemicals.
| Color/physical appearance | Odorless, Colorless, Tasteless |
| Melting point/freezing point | 161.40 K (−111.75 °C, −169.15 °F) |
| Boiling point | 165.051 K (−108.099 °C, −162.578 °F) |
| Density | 5.894 g/L ( at STP) 2.942 g/cm3 (When liquid at B.P) |
| Malleability | No |
| Ductility | No |
| Electronegativity | 2.60 (Pauling Scale) |
Chemical Reaction of Xenon
- The Reaction of Xenon With Water
There is no chemical reaction between Xe and water, however, it does dissolve slightly (108.1 cm3/kg at 20 °C).
- The Reaction of Xenon With Air
Xe does not react with air.
- The Reaction of Xenon With Halogens
It’s possible to get xenon, Xe, to combine with fluorine, F2.
Xe (g) + 2 F2 (g) → XeF4 (s), Combine gases at 400°C and bring down to -78°C.
Xe (g) + F2 (g) → XeF2 (s)
Xe (g) + 3 F2 (g) → XeF6 (s)
Fluorides of Xe are utilized in the production of many other xenon compounds.
- The Reaction of Xenon With Acids
Xe does not react with acids.
- The Reaction of Xenon With Bases
Xe does not react with bases.
Applications of Xenon
Even though Xe is more costly than other gases due to its rarity in the Earth’s atmosphere, it has found considerable application. Here we are discussing the important use of Xe.
Used in Lights and Optics
- Xe is utilized in the production of camera flashes and other types of strobe lights for usage in photography and club lighting.
- In lasers, Xe is utilized to raise the optical gain via excitation of the active medium, which then results in the production of coherent light. The first ever solid-state laser had a Xe-flash lamp for its pumping source.
- Additionally, film projection technologies such as IMAX make use of xenon in their processes. In order to illuminate the massive IMAX displays, projectors make use of xenon bulbs that have the capacity to draw up to 15000 Watts of power.
- Xe lamps produce very high-quality ultraviolet rays with short wavelengths and powerful near-infrared light, both of which are essential components in the production of technologies used for night vision.
- On plasma televisions, xenon is an essential component. Each cell in a plasma display is composed of a combination of Xe and neon, while the display as a whole is made up of plasma. Each time this plasma interacts with the electrodes, ultraviolet photons are produced. These photons, in turn, stimulate the phosphor coating that is located on the front of the displays.
Uses In Medical Science
- Even though it is more expensive than other options, Xe is frequently utilized in the field of anesthesia. Additionally, it is employed as a neuroprotective and cardioprotective agent.
- By causing an increase in the number of red blood cells that are produced within the body, the inhalation of a mixture of xenon and oxygen can make a human being more athletically productive. Despite this, it is a banned chemical and is considered to be a form of cheating in athletic competition.
Health Effects of Xenon
- Xe is considered to be simple asphyxiant even though it is inert. When inhaled in high enough amounts, it can cause vertigo, nausea, vomiting, loss of consciousness, and ultimately death. Errors in judgment, bewilderment, or loss of consciousness that prohibit an individual from rescuing himself could lead to death. When there is a lack of oxygen in the blood, passing out and mortality are possible in a matter of seconds without any prior warning.
Environmental Effects of Xenon
- Because of its low abundance in the atmosphere, Xe is a chemically inert and non-toxic element. The extremely low temperature (-244 degrees Celsius) will cause organisms to freeze upon interaction, but there are unlikely to be any long-term ecological repercussions.
References
- Emsley, John (2011). Nature’s Building Blocks: An A–Z Guide to the Elements (2nd ed.). Oxford: Oxford University Press. ISBN 978-0-19-960563-7.
- Greenwood, N. N.; Earnshaw, A. (1998). Chemistry of the Elements (2nd ed.). Oxford: Butterworth Heinemann. ISBN 0-7506-3365-4.
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.121. ISBN 1-4398-5511-0.
- livescience.com/37504-facts-about-xenon.html
