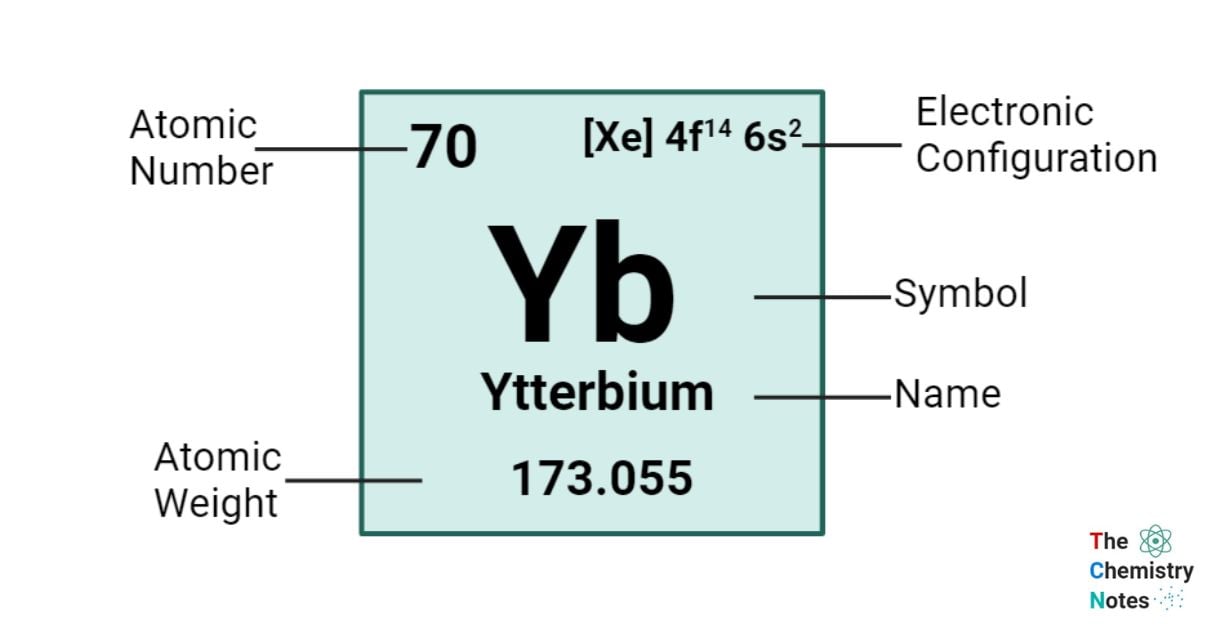
Ytterbium is a chemical element with an atomic number of 70 and is represented by the symbol ‘Yb’ in the periodic table. It is hard and silvery-white with a pale yellow tint in appearance and classified as rare earth metal and belongs to the f-block of the lanthanide group of the periodic table. This particular element holds the position of being the fourteenth and second-to-last element in the lanthanide series. The relative stability of its +2 oxidation state is primarily attributed to this element. Similar to the remaining lanthanides, the prevailing oxidation state of this element is +3, as observed in its oxide, halides, and other chemical compounds.
It is not naturally occurring in its elemental form but rather exists in various minerals, predominantly monazite, gadolinite, euxenite, and xenotime. The Earth’s crust contains approximately 3 milligrams per kilogram of ytterbium.
Interesting Science Videos
History of Ytterbium
- The element was first discovered by Jean Charles Galissard de Marignac in the year 1878, in the city of Geneva, Switzerland.
- Yb nitrate was subjected to thermal treatment, resulting in its decomposition. Subsequently, the remaining material was extracted, yielding an unidentified white powder. This powder was designated as ytterbium oxide, also known as ytterbia.
- In the year 1878, Charles Marignac conducted an experiment involving erbium at the University of Geneva. Through the process of heating erbium nitrate, Marignac successfully isolated Yb, resulting in the formation of red erbium oxide and a pale-colored element that he subsequently designated as ytterbium.
- The synthesis of ytterbium metal was initially achieved in 1937 through a chemical reaction conducted by Klemm and Bonner. This process involved the application of heat to a mixture of ytterbium chloride and potassium.
- In 1953, a group of esteemed researchers, namely David Dennison, A. Daane, and Frank Spedding, successfully synthesized a highly refined iteration of ytterbium metal. This groundbreaking achievement took place at the renowned Ames Laboratory, located in Iowa.
- The element’s nomenclature is derived from its association with Ytterby, a locality situated in Sweden. This town has lent its name to four elements, namely yttrium, terbium, erbium, and four others.
Occurrence of Ytterbium
- Ytterbium, an element found within the Earth’s crust, is present at an estimated concentration of approximately 3 milligrams per kilogram.
- Yb does not occur naturally in its elemental state; instead, it is found in diverse minerals, primarily monazite, gadolinite, euxenite, and xenotime.
- Throughout history, the process of isolating rare earth elements from one another has posed significant challenges and incurred substantial costs due to their closely resembling chemical properties.
- The advent of ion exchange and solvent extraction techniques since the 1940s has yielded substantial reductions in production costs.
- Yb, an element of the periodic table, exhibits remarkable diversity in its isotopic composition. Specifically, it encompasses a total of 30 distinct isotopes, each characterized by a unique number of neutrons within its atomic nucleus. These isotopes span a range of mass numbers, extending from 151 to 180. Furthermore, the half-lives of these isotopes have been meticulously determined and documented, contributing to our understanding of the decay processes inherent to ytterbium’s atomic structure. Ytterbium in its natural state manifests as a composite amalgamation of no less than seven distinct isotopes.
Isotopes of Ytterbium
Yb, in its natural state, is observed to exist as a composite amalgamation comprising a minimum of seven distinct isotopes that include: 168Yb, 170Yb, 171Yb, 172Yb, 173Yb, 174Yb, and 176Yb.
Naturally Occurring Isotopes of Ytterbium
| Isotopes | Natural Abundance (%atoms) |
|---|---|
| 168Yb | 0.1 |
| 170Yb | 3.0 |
| 171Yb | 14.3 |
| 172Yb | 21.8 |
| 173Yb | 16.1 |
| 174Yb | 31.8 |
| 176Yb | 12.8 |
Elemental Properties of Ytterbium
| Electronic Configuration | [Xe] 4f14 6s2 |
| Atomic Number | 70 |
| Atomic Weight | 173.04 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Lanthanides, 6, f-block |
| Density | 6.97 g/cm3 at 20 °C |
| Appearance | silvery-white |
| Van der Waals radius | unknown |
| Electron shells | 2, 8, 18, 32, 8, 2 |
| Electrons | 70 |
| Protons | 70 |
| Neutrons in the most abundant isotope | 104 |
Physical Properties of Ytterbium
- Yb has an atomic number of 70 and is a silvery-white with yellow tint appearing rare earth metal. It has a melting point of 824 °C (1515 °F) and a boiling point of 1196 °C (2185 °F).
- Yb has a solid phase density of 6.97 g/cm3 and a liquid or molten phase density of 6.21 g/cm3.
- It is malleable which means it can be easily beaten into thin sheets without any cleavage.
- It is ductile which means it is possible to draw thin wires from it without breaking.
- Yttrium exhibits three distinct allotropes and undergoes phase transformations at approximately -13°C and 795°C. The precise transition temperature is contingent upon variations in both pressure and stress.
- Ytterbium exhibits paramagnetic behavior at temperatures exceeding 1.0 K (-272.15 °C), in contrast to the remaining rare-earth metals that display antiferromagnetic and/or ferromagnetic characteristics at lower temperatures.
| Color/physical appearance | metallic, silvery-white |
| Melting point/freezing point | 1097 K (824 °C, 1515 °F) |
| Boiling point | 1469 K (1196 °C, 2185 °F) |
| Density | 6.97 g cm-3 at 20° |
| Malleability | Yes |
| Ductility | Yes |
| Electronegativity | 1.1 (Pauling Scale) |
Chemical Properties of Ytterbium
- Ytterbium exhibits a higher degree of reactivity compared to other elements within the lanthanide series.
- Yb exhibits a high propensity for oxidation when exposed to atmospheric oxygen. Typically, it is stored within hermetically sealed containers to prevent its interaction with atmospheric oxygen.
- Yb exhibits a gradual oxidation process when exposed to atmospheric conditions, resulting in the manifestation of a distinctive golden-brown coloration.
- Yb exhibits a sluggish reaction when exposed to water, while its reactivity is notably enhanced when it comes into contact with acids and liquid ammonia.
- Yb exhibits reactivity with Hydrogen (H), resulting in the formation of a diverse range of non-stoichiometric hydride compounds.
- Yb exhibits reactivity with halogens, resulting in the formation of trihalides specific to each halogen.
- Yb exhibits reactivity when exposed to water, with a relatively sluggish reaction observed in cold-water conditions and a more rapid reaction observed in hot-water conditions, resulting in the formation of ytterbium(III) oxide.
- Ytterbium exhibits rapid and facile reactivity when subjected to acid environments, resulting in its dissolution.
Chemical Reaction of Ytterbium
- The Reaction of Ytterbium With Air
Ytterbium metal undergoes gradual oxidation when exposed to atmospheric conditions, resulting in the formation of ytterbium (III) oxide, denoted as Yb2O3, which exhibits a high propensity for combustion.
4 Yb (s) + 3 O2 (g) → 2 Yb2O3 (s)- The Reaction of Ytterbium With Water
Yb exhibits a sluggish reaction rate when exposed to cold water, whereas it demonstrates a rapid reaction rate when subjected to hot water. This reaction results in the formation of ytterbium hydroxide, denoted as Yb(OH)3, along with the liberation of hydrogen gas (H2).
2 Yb (s) + 6 H2O (g) → 2 Yb(OH)3 (aq) + 3 H2 (g)- The Reaction of Ytterbium With Halogens
The element exhibits a propensity to engage in chemical reactions with various halogens, resulting in the formation of ytterbium (III) halides.
The chemical reaction between Yb metal and fluorine gas (F2) results in the formation of ytterbium (III) fluoride, denoted as YbF3.
2 Yb (s) + 3 F2 (g) → 2 YbF3 (s) [White]The chemical reaction between Yb metal and chlorine gas (Cl2) results in the formation of ytterbium (III) chloride, denoted as YbCl3.
2 Yb (s) + 3 Cl2 (g) → 2 YbCl3 (s) [White]The chemical reaction between Yb metal and bromine (Br2) results in the formation of ytterbium (III) bromide, denoted as YbBr3.
2 Yb (s) + 3 Br2 (g) → 2 YbBr3 (s) [White]The chemical reaction between Yb metal and iodine represented as I2, results in the formation of ytterbium (III) iodide, denoted as YbI3.
2 Yb (s) + 3 I2 (g) → 2 YbI3 (s) [White]- The Reaction of Ytterbium With Acid
Yb metal exhibits a high degree of solubility in dilute sulfuric acid, resulting in the formation of solutions that contain the Yb(III) ion in an aquatic form, which is colorless. Additionally, the process of dissolution yields hydrogen gas, denoted as H2. There is a high probability that Yb3+(aq) predominantly exists in the form of the complex ion [Yb(OH2)9]3+.
2 Yb (s) + 3 H2SO4 (aq) → 2 Yb3+ (aq) + 3 SO42- (aq) + 3 H2 (g)Uses of Ytterbium
Numerous significant applications exist for ytterbium and its compounds some of which are discussed here:
Used As Doping Agent
Yb has the potential to serve as a dopant in stainless steel. The incorporation of Yb into stainless steel has been found to enhance its strength, promote grain refinement, and improve various mechanical properties. Yb alloys have been employed in the field of dentistry to a restricted extent.
Used In Atomic Clocks
Yb-based atomic clocks are widely regarded as highly reliable due to their exceptional stability in maintaining precise timekeeping. The level of stability is measured to be within a range of 2 parts per quintillion. An additional laser, operating at a frequency of 518 trillion oscillations per second, induces a modification in the energy states of the atoms. The clocks exhibit a high level of stability as a result of the presence of a significant quantity of atoms.
Used In Lasers
Yb lasers possess the capability to generate short pulses, exhibiting notable durability and efficiency. These devices are particularly utilized in the context of double-clad fibers and solid-state lasers. A laser is an apparatus utilized to generate highly intense light of a singular wavelength. The spectral characteristics of laser light are contingent upon the constituent elements employed in its construction. The inclusion of Yb in the composition of a laser results in distinct properties that distinguish it from a laser without ytterbium.
Used In Radiography
The isotope 169Yb, which has a half-life of 32 days, has been employed as a radiation source in portable X-ray devices in conjunction with the transient 175Yb isotope (half-life of 4.2 days) generated through neutron activation during the irradiation of Yb within nuclear reactors. Similar to X-rays, the emission of gamma rays from the source allows for their transmission through the soft tissues of the body while encountering obstruction from bones and other materials with high density.
Miscellaneous Uses
- Furthermore, it is employed in various industrial applications as a catalyst.
- Yb is a metallic element characterized by its electropositive nature and silvery-white appearance. Upon contact with water, it undergoes a chemical reaction resulting in the formation of ytterbium hydroxide.
- Yb is currently employed in the production of memory devices and tunable lasers.
Health Effects of Ytterbium
- Yb does not possess any discernible biological function; however, it has been observed that its salts have the capacity to enhance metabolic activity.
- Yb has been identified as a substance capable of causing irritation to the skin and eyes, and it is also under suspicion for its potential teratogenic effects.
- It is imperative to store all compounds in sealed containers, ensuring protection from exposure to air and moisture, while also regarding them as substances of high toxicity.
Environmental Effects of Ytterbium
- The presence of metallic ytterbium dust presents a potential risk of fire and explosion. Ytterbium does not present any discernible harm to the flora and fauna, and its salts are being incorporated into the chemical industry as catalysts, replacing those that are deemed hazardous and environmentally deleterious.
References
- www.lenntech.com/periodic/elements/yb
- archive.org/details/naturesbuildingb0000emsl
- www.rsc.org/periodic-table/element/70/ytterbium
- nucleonica.com/index.aspx?ReturnUrl
- hal.in2p3.fr/in2p3-00020241/document
- https://pubchem.ncbi.nlm.nih.gov/element/Ytterbium
- https://www.webelements.com/ytterbium/
- https://www.thoughtco.com/ytterbium-facts-yb-element-606619

