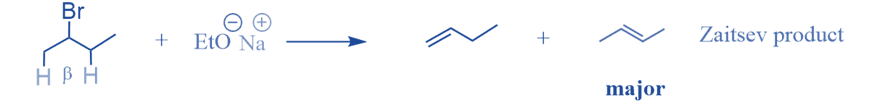
Zaitsev’s rule is an empirical rule that can predict the major alkene product in an elimination reaction. It is also known Saytzeff Rule or Z-rule. Alexander Zaitsev, a Russian chemist, examined many elimination processes and discovered a common pattern in the resultant alkenes. Based on this study, Zaitsev concluded that a stable alkene is created when hydrogen is removed from β-carbon with a small number of hydrogen substituents.
According to Zaitsev, the greatest quantity of alkene generated corresponds to the removal of hydrogen from a β-carbon with the fewest hydrogen substituents. In other words, the thermodynamically most stable product will have the most substituted carbon in the C=C pi bond.
When more than one feasible elimination product exists, Zaitsev’s Rule is used to determine the major elimination product. The elimination processes i.e., dehydrohalogenations result in an alkene. Dehydrohalogenation entails the formation of a pi bond while removing a hydrogen atom and a halogen atom from nearby carbon atoms in a molecule. The alpha carbon is the carbon atom that is linked to the halogen. All nearby carbon atoms are referred to as beta carbons, while the associated hydrogen atoms are referred to as beta hydrogens.
According to Zaitsev’s Rule, when there are multiple alternative beta carbons that can be deprotonated during an elimination reaction, the more substituted one is selected. The more substituted beta carbon will be deprotonated, resulting in a more substituted alkene. The Saytzeff rule is also followed by the Chugaev reaction and pyrolysis of esters in the liquid phase.
Interesting Science Videos
Alkene: Stability
The general idea behind alkene stability is that the more substituted the alkene, the more stable the alkene. An alkene’s C=C double bond has two sp2 hybridized carbon atoms. Aside from their double bond, they are both connected to two other atoms. This alkene is known as tetra-substituted if the four additional atoms to which these two sp2 hybridized carbon atoms are bound are all carbon atoms. If all four of these atoms are hydrogen atoms, this alkene is said to be unsubstituted, which means it has the least degree of substitution.
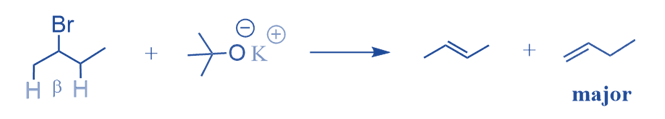
Anti- Zaitsev’s rule
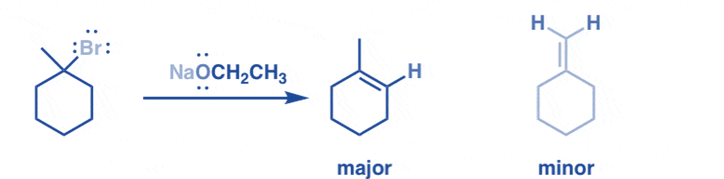
When the identical alkyl halide is treated with a sterically hindered base, the less substituted alkene is the predominant result, although it is less stable.
This is known as Hoffman’s rule, which asserts that if we treat the alkyl halide with a strong sterically hindered base, the less substituted alkene will be the predominant result. This is because the bulky base can more easily access protons that are not inhibited by other carbons. Since the protons of methyl groups are easier to access than those of the CH2 group, the less substituted alkene develops more quickly and is the main byproduct of this reaction.
Factors That Affect Zaitsev’s Rule
Several factors can influence the implementation of Zaitsev’s rule in dehydrohalogenation reactions of alkyl halides. The factors that follow are:
Basicity of The Base
- The basicity of the base utilized in the reaction can also have an impact on how the rule is applied.
- Stronger bases can promote the creation of the less substituted alkene as the main product by encouraging the removal of the hydrogen atom from the carbon atom with more hydrogen atoms bonded to it.
Temperature
- Increasing the temperature of the reaction can also have an effect on its selectivity.
- At increasing temperatures, the energy of the transition state leading to the production of the more substituted alkene increases, favoring its creation.
Steric Hindrance
- At increasing temperatures, the energy of the transition state leading to the production of the more substituted alkene increases, favoring its creation.
- This can lead to the creation of the less substituted alkene as the main product.
Substrate Structure
- The structure of the substrate can also influence how the rule is applied.
- In cyclic systems, for example, the synthesis of the less substituted alkene may be preferred due to the strain caused by the cyclic structure.
Application of Zaitsev’s Rule
Zaitsev’s rule has several applications in organic chemistry. Here are some of its most important applications:
Pharmaceutical Synthesis
It can be utilized in pharmaceutical synthesis, where reaction selectivity is critical for generating medicines with the necessary pharmacological characteristics.
Designing New Molecules
The ability to forecast the result of reactions using the rule can help in the construction of novel compounds with specified features.
Predicting the Product From Reactions
- Chemists may use the rule to forecast the primary result of a dehydrohalogenation reaction, which is useful for developing and optimizing synthetic pathways.
- The regioselectivity of the olefin generated by the elimination process of 2o or 3o alkyl halides is predicted by Saytzeff’s rule.
Understanding Reaction Processes
It aids scientists in understanding the mechanics of dehydrohalogenation processes, which is critical for designing novel reaction pathways and refining current ones.
Synthesizing Complex Natural Products
The rule can preferentially produce highly substituted alkenes during synthesis, and many natural compounds include these alkenes.
Limitation of Zaitsev’s rule
- This criterion does not apply to the gas-phase pyrolysis of esters and the elimination of onium salt. Such a reaction follows the Hofmann rule, which specifies that the products have the fewest substituted olefins.
- The product may follow the Hofmann rule due to steric impact during the elimination phase.
- The size of the base employed in the elimination affects Zaitsev’s rule of regioselectivity.
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s Advanced Organic Chemistry: Reactions Mechanisms and Structure (5th ed.). Wiley.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992. production of polyesters, polyurethanes, and alkaline resins.
