Chemical kinetics examines the rate and mechanism of chemical processes in terms of reactants and products. The Order of reaction describes the relationship that exists between the rate of a chemical reaction and the concentration of the elements involved in it.
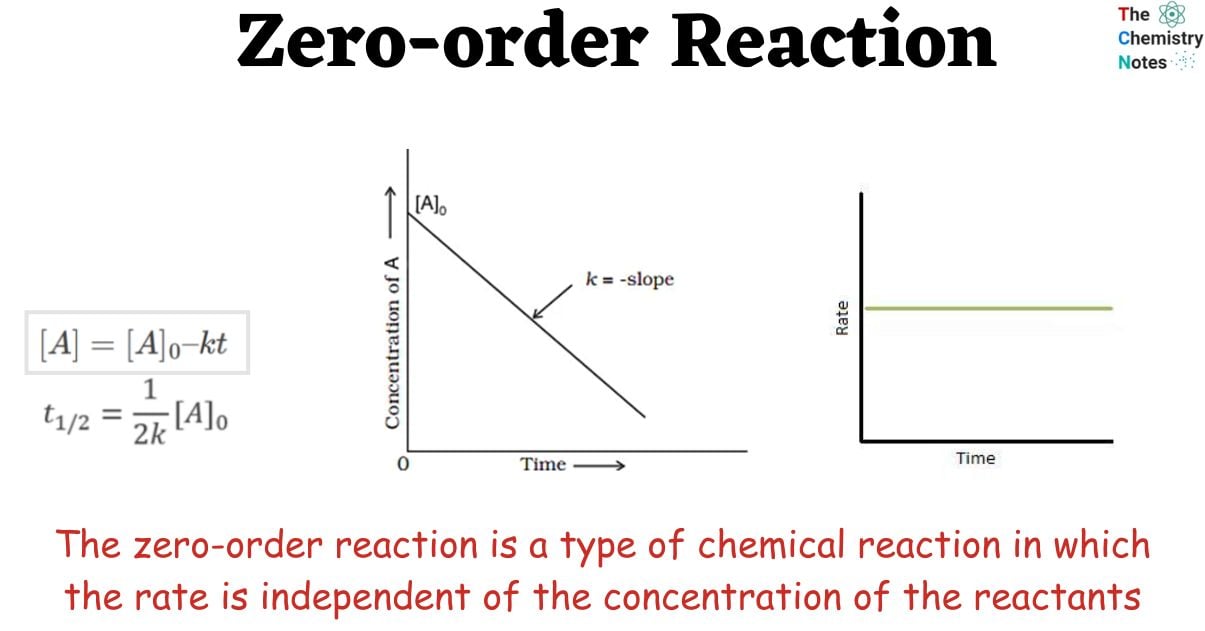
The zero-order reaction is a type of chemical reaction in which the rate is independent of the concentration of the reactants. Since the rate of these reactions is proportional to the concentration of the reactants, the rate of these reactions is always equal to the rate constant of the particular reactions.
Interesting Science Videos
Differential and Integrated Rate Equation of Zero-Order Reaction

The Differential form of a zero-order reaction can be written as:
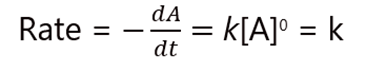
Where ‘Rate’ refers to the rate of the reaction and ‘k’ is the rate constant of the reaction.
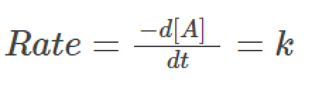
Multiplying both sides with ‘-dt’, we get:

Integrating on both sides, we get:
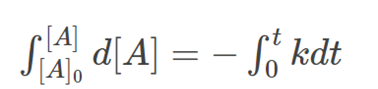
Where [A]0 is the initial concentration of the reactant [A] at time t=0. Solving for [A], we get:
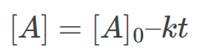
Which is the required integral form. This form enables us to calculate the population of the reactant at any given time post the start of the reaction.
Characteristics of Zero-Order Reactions
- The concentration of the reactant has no effect on the rate of a zero-order reaction.
- The concentration of product increases linearly with time.
- The rate constant is equal to the rate of reaction at all concentrations.
- The unit of zero-order reaction is mol L-1sec-1
Graph of Zero-Order Reaction
The integral form of zero-order reactions can be compared with that of a straight line (y = mx + c), and an [A] against t graph can be plotted to get a straight line with a slope equal to ‘-k’ and intercept equal to [A]0 as shown below.
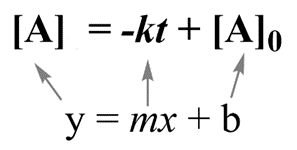
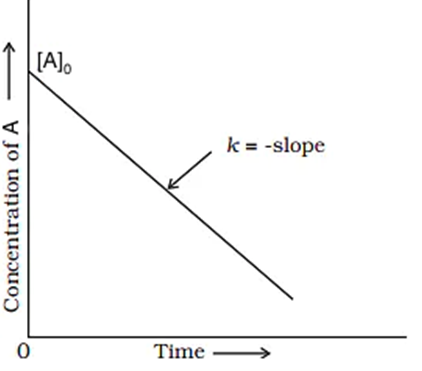
We can get the graph shown below by plotting the rate as a function of time. This only covers a limited span of time. The rate constant, k, is equal to the graph’s slope. K is therefore a constant across time. Furthermore, we can see that the reaction rate is fully independent of the amount of reactant added.
![Rate vs Time [Zero-order reaction]](https://scienceinfo.com/wp-content/uploads/2023/06/image-379.png)
The half-life of Zero-order Reaction
The half-life of a reaction is the time frame in which a reactant’s concentration is reduced to one-half of its initial concentration. It can be calculated as follows
From the integral form, we have the following equation
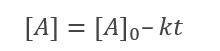
At half-life, t = t1/2 and [A] = [A]o/2. Substituting these values in the equation for concentration, we get
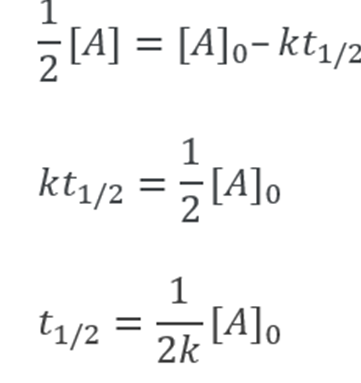
It can be noted from the equation given above that the half-life is dependent on the rate constant as well as the reactant’s initial concentration.
Units of a Zero-Order Reaction
Zero-order indicates that the rate does not depend on the concentration, and therefore, the rate is equal to the concentration.
rate = k[A]0
[A]0 = 1, therefore,
rate = k
The units for the rate are mol/L, so it is the same as the rate constant:
k = mol/L s or M/s or M s-1
Examples of Zero-Order Reaction
The following reactions are examples of zero-order that are not dependent on the concentration of the reactants. Reactions, wherein a catalyst is required (and is saturated by reactants), are generally zero-order.
- The reaction of hydrogen with chlorine (Photochemical reaction).
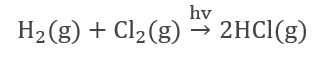
- Decomposition of nitrous oxide over a hot platinum surface.
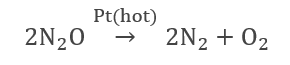
- The decomposition of NH3 in the presence of molybdenum or tungsten is a zero-order reaction.
2NH3 → N2 + 3H2
- Iodization of Acetone (In H+ ion-rich medium)
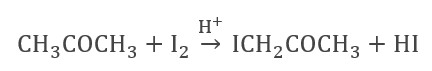
Enzyme-Catalyzed Reaction
Enzymes are complex proteins created by living creatures in our environment. These are responsible for catalyzing an infinite number of chemical changes in our living system. Catalase, generated from living plant cells, for example, catalyzes the decomposition of hydrogen peroxide. Maltase, which converts maltose to glucose, and zymase, which converts glucose to ethyl alcohol, are two enzymes found in yeast. All reactions will proceed in zero order with respect to the substrate for a given amount of enzyme.
Frequently Asked Questions (FAQ)
What is the Zero-Order Reaction?
Zero-order reactions are those in which the concentration of the reactants does not change over time and the concentration rates remain constant.
What is the rate constant unit of Zero Order Reaction?
The rate constant of a zero-order is given as concentration/time or M/s, where ‘M’ is the molarity and ‘s’ is one second.
The rate constant unit is k = mol L-1 s-1.
What does a Zero-order graph look like?
The graphical representation of the concentration as a function of time for a zero-order reaction exhibits a linear relationship characterized by a negative slope.
How to identify zero-order reactions?
By examining the rate of reaction, zero-order reactions can be quickly identified. These reactions proceed at a consistent rate the entire time. The rate of the reaction is determined by varying the concentration of the reactant, and if the rate is found to be constant for all concentrations, the reaction is referred to as a zero-order reaction.
Video on Zero-order Reaction
References
- https://byjus.com/chemistry/zero-order-reaction/
- https://www.toppr.com/guides/chemistry/zero-order-reaction/#
- https://www.vedantu.com/chemistry/zero-order-reaction
- https://classnotes.org.in/class12/chemistry12/chemical-kinetics/integrated-rate-expression/
- Petrucci, Ralph H., William S. Harwood, Geoffrey Herring, and Jeffry D. Madura. General Chemistry: Principles & Modern Applications. Ninth ed. Upper Saddle River, N.J.: Pearson Education, 2007. Print.
- https://collegedunia.com/exams/zero-order-reaction-chemistry-articleid-1318

