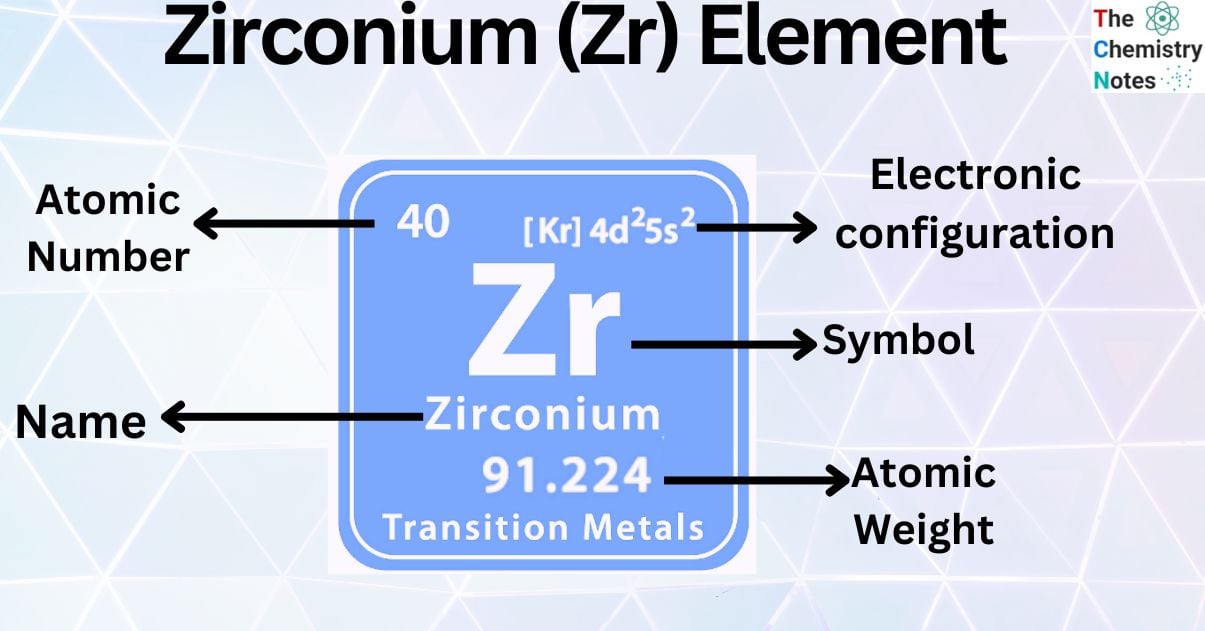
Zirconium is a metallic element with the atomic number 40 and is represented by the symbol ‘Zr’ in the periodic table. It is classified as a transition metal and belongs to the d-block of group 4 of the periodic table. With almost 0.016 percent of presence on the Earth’s crust, it is considered the 18th most abundant element on Earth’s crust.
It is a grayish-silvery, lustrous metal that is found in very small quantities only in the form of crystals. Zirconium is mostly found in silicate-intrusive rocks like granite. It is only found in a few minerals, most notably zircon (ZrSiO4), Baddeleyite (ZrO2), and the rarer red eudialyte (Na4 (Ca Ce Fe Mn)2 ZrSi6O17(OH Cl)2).
Interesting Science Videos
History of Zirconium
- Martin Heinrich Klaproth, a German chemist, discovered a new element in a jargoon from the island of Ceylon (now Sri Lanka) in 1789.
- Klaproth named the newly found element zirconia.
- Humphry Davy, a British chemist, attempted to isolate this new element in 1808 through electrolysis but failed.
- In the year 1824, Jons Jacob Berzelius, a Swedish chemist, was the first to obtain an impure form of zirconium metal. It was done by heating a mixture of potassium zirconium fluoride and potassium in an iron tube.
- The element zirconium gets it name from an arabic word “zargun”,meaning golden colored gemstone.
Occurrence of Zirconium
- With almost 0.016 percent of Zirconium presence on the Earth’s crust, it is considered the 18th most abundant element on Earth’s crust.
- Zirconium is mostly found in silicate intrusive rocks like granite. It occurs only in a few minerals, most notably zircon (ZrSiO4), Baddeleyite (ZrO2), and the rarer red eudialyte (Na4(CaCeFeMn)2ZrSi6O17(OHCl)2).
- Secondary deposits, sometimes known as soap deposits, are often utilized as raw materials. These form when the surrounding rock weathers and only the weather-resistant zircon remains.
- Other similar deposits can form as a result of water currents washing out the zirconium crystals and depositing them elsewhere. Primary deposits, on the other hand, typically have zirconium concentrations that are insufficient for successful mining.
- Australia, Brazil, India, Russia, South Africa, and the United States are the major producer of zirconium among others.
Isotopes of Zirconium
Five stable isotopes of zirconium occur naturally: 90Zr, 91Zr, 92Zr, 94Zr, and 96 Zr.
Natural Isotopes Of Zirconium
| Isotopes | Natural abundance (atom %) |
|---|---|
| 90Zr | 51.45 (40) |
| 91Zr | 11.22 (5) |
| 92Zr | 17.15 (8) |
| 94Zr | 17.38 (28) |
| 96 Zr | 2.80 (9) |
Elemental Properties of Zirconium
| Electronic Configuration | [Kr] 4d25s2 |
| Atomic Number | 40 |
| Atomic Weight | 91.224 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 4, 5, d-block |
| Density | 6.52 g.cm -3 at 20 °C |
| Ionic radius | 0.08 nm (+4) |
| Van der Waals radius | 0.160 nm |
| Electron shells | 2,8,18,10,2 |
| Electrons | 40 |
| Protons | 40 |
| Neutrons in most abundant isotope | 50 |
Physical Properties of zirconium
- Zirconium has an atomic number of 40 and is a silvery-gray metal. It has a melting point of 1854°C (3369°F) and a boiling point of 4406°C (7963°F).
- Zr has the density of 6.52 grams per cubic centimeter.
- Zr can also be in powder form which is black in color and highly flammable.
- It is malleable, allowing it to be easily hit into sheets without cleavage, and ductility, which makes it possible to draw thin wires from it.
- It is highly resistant to corrosion and heat.
| Color/physical appearance | Lustrous, metallic, greyish tinge |
| Melting point/freezing point | 1854°C, 3369°F, 2127 K |
| Boiling point | 4406°C, 7963°F, 4679 K |
| Density | 6.52 g cm-3 at 20° |
| Electronegativity | 1.2 (Pauling Scale) |
Chemical Properties of Zirconium
Zirconium is comparatively less reactive element.
Zirconium are diamagnetic in nature.
Chemical Reaction of Zirconium
- The reaction of Zirconium with Air
Zirconium metal is coated with an oxide layer, which usually renders it inert. However, it does burn in the air when exposed to the dioxide zirconium(IV) oxide, ZrO2.
Zr (s) + O2 (g) → ZrO2 (s)
- Reaction of Zirconium with Water
Zirconium does not react with water under normal condition.
- Reaction of Zirconium with the Halogens
Zirconium reacts with halogens to form zirconium(IV) halides upon heating
Zirconium reacts with fluorine, F2, to form the halide zirconium (IV) fluoride, ZrF4.
Zr (s) + 2F2 (g) → ZrF4 (s) [white]
Zirconium reacts with bromine, Br2, to form the halide zirconium (IV) bromide, ZrBr4.
Zr (s) + 2Cl2 (g) → ZrCl4 (l) [white]
Zirconium reacts with chlorine, Cl2, to form the halide zirconium (IV) chloride, ZrCl4.
Zr (s) + 2Br2 (g) → ZrBr4 (s) [white]
Zirconium reacts with iodine, I2, to form the halide zirconium (IV) iodide, ZrI4.
Zr (s) + 2I2 (g) → ZrI4 (s) [white]
- Reaction of Zirconium with Acids
Zirconium metal is frequently covered with an oxide layer, which renders it inert. The majority of cold mineral acids have no effect. Zirconium dissolves in hydrofluoric acid (HF), most likely to produce fluoro complexes.
Uses of Zirconium
Zirconium can be used in different types of application some of which are discussed below:
Used In Ceramics Industry: Zircon sand is used in ceramic glaze due to its chemical stability. It is also used for building ceramics, sanitary ceramics, and the daily ceramic industry. The major component of zircon sand is zirconium silicate. Zircon sand is transformed by grinding, sintering, and polarization in the ceramics.
It is primarily utilized in the creation of colored glaze for architectural ceramics, daily-use ceramics, and electric porcelain as a high-quality and low-cost ceramic glaze opacifier.
Used In Ammunition Industry: Sponge zirconium powder is extremely combustible and is mostly used to make flash powder and military-grade blowing agents. Higher quality zirconium is classified as nuclear grade and is utilized in airplanes, submarines, and nuclear reactor components.
Used In Nuclear Reactors: Zirconium does not corrode quickly, which implies it doesn’t deteriorate quickly when exposed to oxygen or water. This means that it can be used to produce a variety of useful products without having to be changed on a regular basis. As a result, zirconium is utilized in nuclear reactors, which generate atomic energy. Approximately 90% of all zirconium produced each year is utilized to produce nuclear power.
Used In Superconducting Magnet: Zirconium is also used to make superconducting magnets, which are electromagnets that generate power. Zirconium is most likely present in your television’s glass, and photographers use zirconium whenever they use a flashbulb to take a photograph.
Used In Manufacturing Industries: Because zirconium has a high melting point, it can be employed in the manufacturing industry. Zirconium is mixed with other metals to form crucibles, which are receptacles where other metals can be melted. Furthermore, zirconium is utilized to create bricks, scissors, knives, food packaging, and microwave oven parts.
Used In Jewelry: Gemstone zircon can be found in a variety of colors; one of them has a golden hue, which is used to make jewelry. Cubic zirconia appears colorless, but it is used as a synthetic gemstone. It can resemble a diamond when cut into a design.
Used In Medical Science: This chemical is utilized in the manufacture of a range of biomedical products. It comprises knee and hip replacement parts, dental implants and crowns, and a variety of other prosthetic and restorative devices. The propensity of zirconium to bond with urea has been well-known. As a result, it is commonly used to treat chronic renal illness.
Health Effects Of Zirconium
- In general, zirconium and its salts have little systemic toxicity. The average dietary intake is around 0.05 mg.
- A significant amount of zirconia moves through the gut without being adsorbed, and what gets absorbed accumulates significantly more in the skeleton than in tissue.
- 95 Zr is a radioactive isotope involved in nuclear testing and is one of the long-lived radionuclides that cause cancer.
Environmental Effects Of Zirconium
Research conducted by different scientists and researchers has not shown any significant effects of zirconium in the environment until now.
- Zr’s mobility and phytoavailability in soil are influenced by both its speciation and the physicochemical characteristics of the soil, such as its pH, texture, and organic content.
- Zr is primarily accumulated in root cells after plant absorption. There is no known vital function for Zr in plant or animal metabolism. Despite the scarcity of published evidence, we find that Zr’s phytotoxicity is generally modest. Regardless, Zr can severely limit plant growth and influence plant enzyme activity.
- Because Zr may have an impact on the biosphere, we feel it deserves to be studied in additional studies that will improve our understanding of its behavior in soil-plant systems.
References
- Nielsen, Ralph (2005) “Zirconium and Zirconium Compounds” in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim. doi:10.1002/14356007.a28_543
- https://www.lenntech.com/periodic/elements/zr.htm
- https://www.rsc.org/periodic-table/element/40/zirconium
- https://www.chemicool.com/elements/zirconium.html
- Mary Elvira Weeks, The Discovery of the Elements XI., Journal of Chemical Education., July 1932, p1231/2.
- Considine, Glenn D., ed. (2005). “Zirconium”. Van Nostrand’s Encyclopedia of Chemistry. New York: Wylie-Interscience. pp. 1778–1779. ISBN 978-0-471-61525-5.
- Edward Turner, Franklin Bache, Elements of Chemistry: Including the Recent Discoveries and Doctrines of the Science, 1830, John Grigg, p304/5.
- https://pubmed.ncbi.nlm.nih.gov/1283692/

