Berkelium is a synthetic chemical element with an atomic number of 97 and is represented by the symbol ‘Bk’ in the periodic table. It is silvery in appearance and belongs to the f-block of period 7 of the periodic table. Berkelium was identified as the fifth synthetic trans-uranium element in the actinide series. Similar to other elements in the actinide class, berkelium exhibits significant radioactivity. It is an explosive element found in almost all current nuclear weapons. American chemists Glenn T. Seaborg, Joseph W. Kennedy, and Arthur C. Wahl discovered curium in 1944. They created it by bombarding 7 grams of americium-241 with alpha particles in a 152-cm (60-inch) cyclotron in Berkeley, California.
Interesting Science Videos
History of Berkelium
- The Berkeley researchers dissolved the target in acid and then used ion exchange to separate the newly formed components. This was the isotope berkelium-243, which has a half-life of around 5 hours.
- Stanley Thompson, Albert Ghiorso, and Glenn Seaborg are credited with discovering Berkelium in 1949, after bombarding Americum-241 with alpha particles for several hours in a 60-inch cyclotron.
- Burris Cunningham and Stanley Thompson isolated beryllium in higher quantities in 1958 using a five-year neutron irradiation of plutonium-239 at the Materials Testing Reactor in Arco, Idaho.
- Nine more years passed before enough berkelium was produced—just a few micrograms—to be visible to the unaided eye.
- The first measurable amounts of berkelium chloride (BkCl3), a chemical containing berkelium, were created in 1962 and weighed roughly 0.000000003 grams, or 3 billionths of a gram.
- The element was named after Berkeley, California, the location where it was initially created. The element’s nomenclature corresponded with that of other actinides, with names that follow the sequence of the lanthanide elements located above them in the periodic table.
Occurrence of Berkelium
- Berkelium is found on Earth in regions in which atmospheric nuclear testing have been undertaken or in locations where a nuclear calamity has occurred.
- Berkelium, as well as other actinides, was discovered in large concentrations at the first US hydrogen bomb experiment site.
- Berkelium is created in trace amounts in nuclear reactors like the High Flux Isotope Reactor at Oak Ridge National Laboratory, Tennessee, by bombarding plutonium (Pu), curium, or americium (Am) with alpha particles.
- It is created in nuclear reactors, specifically the isotope berkelium-249.
- Berkelium has 12 isotopes with known half-lives, ranging from mass 232 to 247.
- Berkelium isotopes have a relatively short half-life, implying that any primordial berkelium has decayed by now.
Elemental Properties of Berkelium
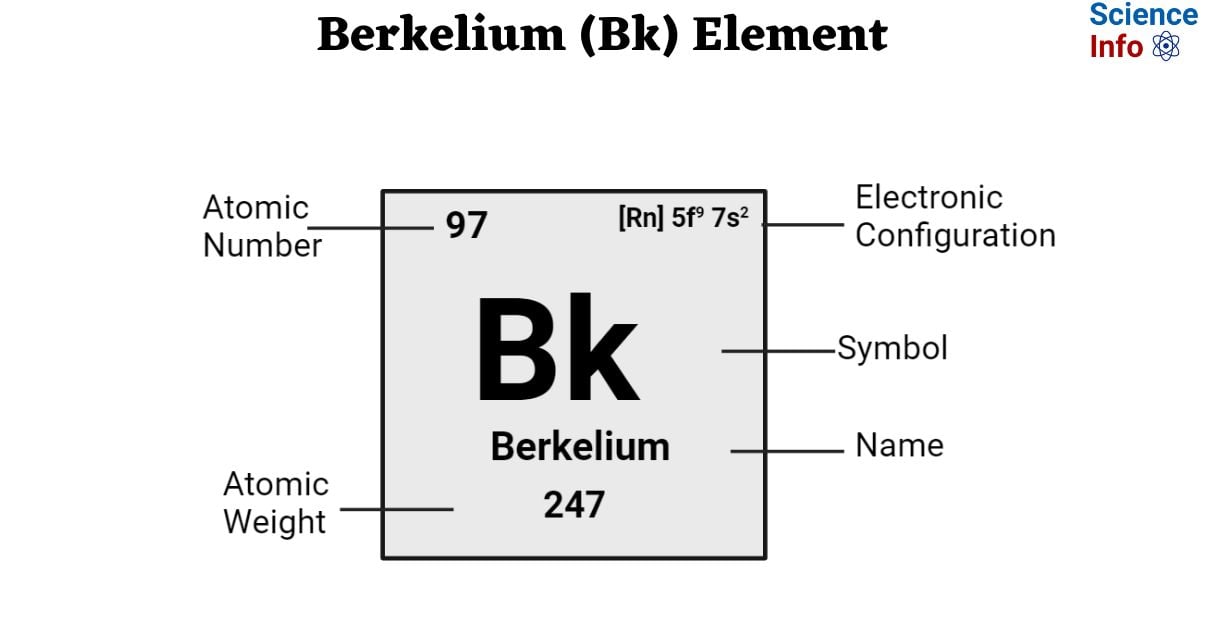
| Electronic Configuration | [Rn] 5f9 7s2 |
| Atomic Number | 97 |
| Atomic Weight | 247 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Actinides, 7, f-block |
| Density | 14.79 g/cm3 at 20 °C |
| Ionic radius | unknown |
| Van der Waals radius | – |
| Electron shells | 2, 8, 18, 32, 27, 8, 2 |
| Electrons | 97 |
| Protons | 97 |
| Neutrons | 150 (Varies with isotopes) |
Isotopic Information of Berkelium
- Berkelium is made up of radioactive isotopes. Berkelium-243 was the first isotope to be synthesized.
- Its most stable isotope is berkelium-247, with a half-life of 1380 years before disintegrating into americium-243 undergoing alpha decay.
- Berkelium comprises 12 isotopes with known half-lives ranging from 232 to 247.
- The longest-lived isotopes are 247-Bk, which has a half-life of 1380 years; 249-Bk, which has a half-life of 320 days; and 245-Bk, which has a half-life of 4.94 days.
Physical Properties of Berkelium
- Berkelium is a silver-white, soft and extremely radioactive metal.
- It has a density and melting point that fall somewhere between curium and Californium. Its melting point is 986 degrees Celsius, and its density is 14.78 grams per cubic centimeter.
- The element exhibits isomerism, with six nuclear isomers being characterized.
- Berkelium is antiferromagnetic at temperatures below 34K and paramagnetic at higher temperatures.
- It is a paramagnetic metal with one of the lowest bulk modulus values among the actinides.
- Berkelium metal is dimorphic, with double hexagonal close packed crystals at ambient temperature and face-centered cubic structures at higher temperatures.
- Under normal conditions, berkelium metal has hexagonal symmetry, which transforms to a face-centered cubic structure at ambient temperature and an orthorhombic structure when compressed to 25 GPa.
- The fluorescence of Bk3+ ions is detected at 652 nanometers (red) and 742 nanometers (deep red).
Chemical Properties of Berkelium
- Its chemical characteristics are similar to those of the neighboring element in the lanthanide class, terbium.
- It dissolves in a wide range of aqueous inorganic acids, as do all other actinides.
- When berkelium dissolves in a range of aqueous inorganic acids, hydrogen is released and berkelium(III) is created.
- In aqueous solutions, +3 is the most stable oxidation state, but it easily adopts +4 in solids.
- Berkelium solution compounds with oxidation states of +2 and +4 are also known, however they are less stable than compounds with an oxidation state of +3.
- Berkelium has been seen to produce an oxide layer on its surface when exposed to air or oxygen. This layer limits additional reactions with oxygen.
- Berkelium develops a protective oxide layer on its surface and does not readily react with oxygen at room temperature, but it does react with molten metals to form numerous binary compounds.
- Berkelium is known to react with halogens, hydrogen, pnictogens, and chalcogens to generate a wide range of binary compounds.
Uses of Berkelium
- Berkelium is now unavailable for commercial or technological usage due to its scarcity.
- Berkelium is mostly used in basic scientific research due to its artificial production and scarcity. It has proven very useful in that regard. Most of this study focuses on the synthesis of heavier elements.
- 249-Bk is a target that is frequently utilized to create heavier elements through charged particle bombardment because of its comparatively long half-life and availability in microgram quantities.
- Berkelium-249 behaves as a nuclide, producing lawrencium, rutherfordium, and bohrium. It can also be used as a source of californium, as berkelium-249 undergoes beta decay to yield californium-249.
- As the first member of the second half of the actinide series, its research can help us gain a better knowledge of the behavior of heavier elements, which can be difficult to study due to scarcity, radioactivity, and other factors.
Health Effects of Berkelium
- It has a short half-life of 330 days and decays into californium-249, a powerful alpha emitter that may be harmful to the human body. Berkelium, if consumed, can absorb in the human body and eventually cause cancer.
- Even modest doses of berkelium are said to cause cancer. Carcinogenic damage tends to accumulate in the gene pool, generating further damage.
Environmental Effects of Berkelium
- It is needless to be concerned about berkelium’s effects on the environment because it does not naturally occur and is not present in the earth’s crust.
- Berkelium is mainly manufactured and managed within controlled laboratory environments, minimizing the likelihood of environmental exposure. The brief half-life of numerous berkelium isotopes also restricts their potential environmental impact. In research facilities, synthetic elements such as berkelium are typically treated with utmost caution to avoid unintended releases into the environment.
Toxicity of Berkelium
- Berkelium’s toxicity has not been thoroughly explored, although it is reasonable to presume that it poses a health risk if consumed or inhaled due to its radioactivity.
- Berkelium-249 emits low-energy electrons and is relatively safe to handle.
- It decays in alpha-emitting californium-249, which is relatively safe to handle but produces free radicals and causes the sample to self-heat.
Video Reference
References
- https://orau.org/health-physics-museum/files/library/berkeliumdiscovery.pdf
- https://periodic-table.com/berkelium/
- https://www.webelements.com/berkelium/
- https://www.rsc.org/periodic-table/element/97/berkelium
- https://www.lenntech.com/periodic/elements/bk.htm
- https://www.chemicool.com/elements/berkelium.html
- https://www.thoughtco.com/berkelium-element-facts-bk-3863126
- https://chemicalengineeringworld.com/berkelium-element-properties-and-information/

