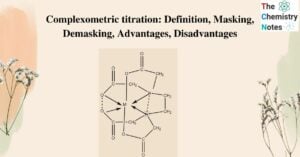
Pearson’s HSAB Principle: Definition, Applications, Limitations
HSAB principle (i.e Hard and Soft Acids and Bases principle) was introduced by Ralph Pearson to explain the stability of metal complexes and the process of their reactions. R. G. Pearson extended and modified the qualitative relationship between Lewis acids … Read more