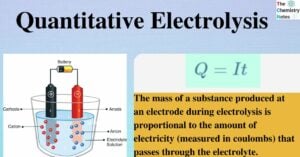
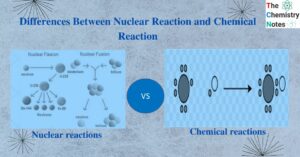
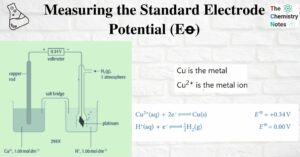
Dot and Cross Diagram
A dot and cross diagram can be used to illustrate how bonds are formed in small molecules. Diagrams called “dot and crosses” display how the outer-shell electrons of an ionic or covalent compound or element are arranged.Dots and crosses are … Read more