Understanding the difference between osmosis and diffusion is important because both are essential concepts in biology, chemistry, and physics that have been seen and researched for many years. These two mechanisms are important in a variety of biological phenomena, including plant water absorption and cell function. By digging deeper into their differences, we gain a better grasp of how life thrives.
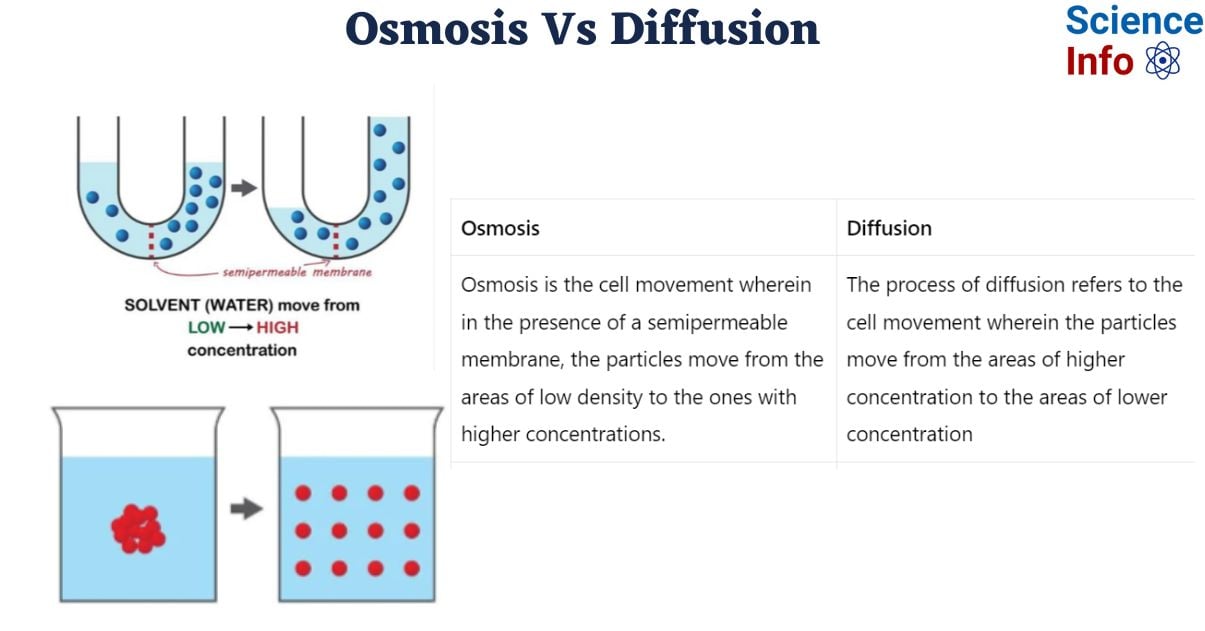
Diffusion is the process by which particles move from areas of high density to areas of low density in order to balance the concentration throughout the medium. Osmosis is the process by which particles pass across a semipermeable membrane from a low-concentration area to a high-concentration area.
Interesting Science Videos
What is Osmosis?
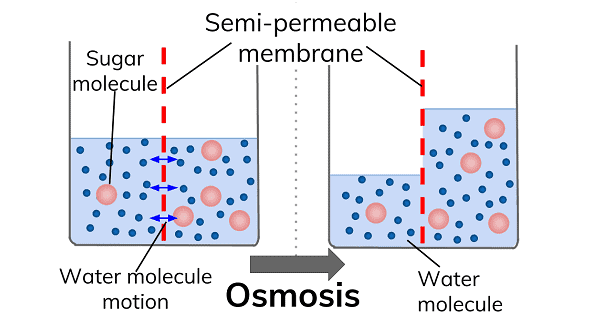
Osmosis is the process by which water molecules migrate from a high-concentration location to a low-concentration area via a semi-permeable membrane. Osmosis occurs across the cellular membrane to keep a cell from becoming flaccid (insufficient water) or turgid (excess water).
Osmosis occurs when a semipermeable membrane separates a solution from a solvent, allowing solvent molecules to pass through and enter the solution. This phenomenon is known as osmosis.
The semi-permeable membrane can be utilized to achieve the same result. In such cases, solvent molecules can pass across the semi-permeable barrier from a lower concentration to a higher concentration solution.
In other terms, osmosis is the spontaneous transfer of solvent molecules onto or from a solution via a semi-permeable membrane.
What is a semipermeable membrane?
Semi-permeable membranes allows the passage of solvent molecules but not solute particles.
The semi-permeable component could be natural or manufactured. Natural semi-permeable membranes include those that wrap plant and animal cells, as well as the pig bladder. Artificial semi-permeable membranes can be made using gelatinous precipitates of inorganic substances such as calcium phosphate and copper ferrocyanide.
Types of Osmosis
There are two types of osmosis: Endosmosis and exosmosis.
- Endosmosis happens when a substance is submerged in a hypotonic solution and the solvent molecules penetrate the cell, causing it to become turgid or undergo deplasmolysis. This is known as endosmosis.
- When a substance is immersed in a hypertonic solution, the solvent molecules leave the cell, causing it to become flaccid or undergo plasmolysis. This is known as exosmosis.
Osmotic Types of Solutions
- Isotonic solution.
- Hypertonic solution.
- Hypotonic solution.
Isotonic Solution
An isotonic solution contains the same number of solutes as another solution. For example, an isotonic cell to the surrounding solution indicates that both the internal fluid and the external fluid have the same osmotic pressure and water potential. In this instance, there will be no net flow of water molecules between the cell and the surrounding fluid.
Hypotonic Solution
A hypotonic solution is one that has a lower osmotic pressure (or contains fewer solutes) than another solution. In this situation, water travels toward the less concentrated zone or toward the more concentrated part to dilute the solution. When the fluid surrounding the cell is hypotonic, for example, water will migrate across the membrane toward the more concentrated solution inside the cell.
Hypertonic Solution
A hypertonic solution appears to be the inverse of a hypotonic solution. A hypertonic solution has more solutes and less water than the other solution. When a cell is submerged in a hypertonic solution, water will exit the cell to dilute the solution outside.
Factors affecting osmosis
Osmosis is caused by a variety of reasons, and so the rate of osmosis is influenced by a number of such factors.
- Temperature: The rate of osmosis increases with the temperature of the system. This occurs because when the temperature rises, the energy of the molecules increases. As the molecules’ energy increases, so does their movement, and the osmosis process accelerates.
- Concentration gradient: Because the concentration of solute molecules is critical to the driving force of osmosis, any changes in concentration have a direct impact on the rate of osmosis. Osmosis increases when the concentration difference across the membrane is greater. As the quantity of solute molecules in one solution increases, the pressure exerted by the solvent molecules decreases, speeding up the osmosis process. The process of osmosis ends when balance is maintained across the membrane.
- Water potential/solvent potential: The water potential across a semi-permeable membrane determines the rate of osmosis. As a solution’s water potential increases, water molecules can migrate across the membrane due to the increasing pressure produced by the particles.
Eventually, the water potentials on either side equalize, resulting in equilibrium.
Once equilibrium is attained, water flows across the membrane in equal amounts both ways, stabilizing the solutions. - Surface area and thickness of the membrane: With an increase in surface area, molecules will have more space to flow, increasing the rate of osmosis. Similarly, reducing the surface area leaves less space for molecules to move, limiting their mobility. The rate of osmosis decreases as the membrane thickness increases.
- Pressure: Pressure is an important component influencing the process of osmosis since it can even shift the direction of osmosis.
If the pressure is greater than the pressure applied by the solvent molecules, the direction of osmosis may change, and the solvent molecules will begin to migrate towards the location with the highest solvent concentration. However, applying pressure less than that exerted by the solvent molecules does not change the orientation, but it does lessen the rate of osmosis.
Pressure applied in the same direction as the concentration gradient improves the rate of osmosis.
Importance of Osmosis
- Osmosis is responsible for the transportation of nutrients into the cell and waste materials out of the cell.
- It absorbs water from the earth and transports it to the plant’s higher sections via the xylem.
- It stabilizes the environment inside a living thing by maintaining a balance of water and intercellular fluid levels.
- Despite the constant loss of water by transpiration, it is a technique by which plants maintain their water content.
- Osmosis creates cell turgor, which determines how much movement is permitted for plants and plant components.
- The balance of water and intracellular fluid levels maintains the interior environment of live cells.
Example of Osmosis
- Plant roots absorb water from the soil by osmosis, which moves water molecules from higher concentrations in the soil to lower concentrations in the plant roots.
- Osmosis helps to regulate blood pressure by balancing the concentrations of solutes in the blood and surrounding tissues. For example, if the concentration of solutes in the blood is too high, water will migrate out of the tissues and into the blood vessels by osmosis, hence increasing blood volume and pressure.
- Osmosis is commonly used to preserve food by preventing bacteria and other microbes from growing. Pickling or curing meat in salt, for example, causes water to exit bacteria and other microbes’ cells, perhaps leading to their death.
- The kidneys use osmosis to filter waste items from the blood and eliminate excess water from the body. The osmotic gradient between the blood and the surrounding tissues regulates the flow of water and solutes through the kidney’s nephron.
What is Diffusion?
Diffusion is the movement of molecules along a concentration gradient. It is an important process that occurs in all living beings. Diffusion facilitates the passage of chemicals into and out of cells. The molecules travel from a location of higher concentration to a region of lower concentration until the concentration is balanced throughout.
Diffusion has applications in a variety of fields. In biology, it controls particle mobility across cell membranes. In industry and chemistry, it affects reactions, heat transport, and materials manufacture.
The diffusion substance may be in any state of matter (solid, liquid, or gas). Molecules move against an electrochemical gradient. It is vital in the cell since it allows it to function properly and perform many tasks. In biology, temperature, concentration gradients, particle size, and medium properties all have an impact on the diffusion process. It helps us understand how chemicals mix and spread.

Types of Diffusion
Diffusion can be divided into two types: simple and facilitated diffusion.
Simple diffusion: Simple diffusion refers to substances passing straight through a semi-permeable barrier without the use of transporters. Some requirements must be met for the process to occur, such as the solute being non-polar and having a molecular weight of less than 10,000 kDa. For example, little bacteria’s nutrition and the movement of carbon dioxide, water, and oxygen in the cytoplasm.
Facilitated diffusion: Facilitated diffusion is described as the movement of substances over a semi-permeable membrane from an area of higher concentration to a region of lower concentration using transport proteins. These proteins function as channels or carriers, allowing chemicals to pass.
Facilitated diffusion can be further divided into two types:
Dialysis is a type of assisted diffusion in which a selective membrane only permits certain molecules or atoms to pass through it.
Osmosis is another type of assisted diffusion in which water molecules pass through a semi-permeable membrane. It occurs as a result of a differential in solute concentration on either side of the membrane, causing water to migrate from one area of lower solute concentration to another of higher solute concentration.
Factors Affecting Diffusion
- Size of the solute molecules: The size of the molecules influences the rate of diffusion over a biological membrane. Large molecules make it more difficult to migrate across the membrane, slowing the rate of diffusion. Thus, diffusion is faster for smaller molecules and slower for larger molecules.
- Temperature: The temperature of the system also influences the process of simple diffusion.
The energy of the molecules rises in proportion to the temperature. Molecules with more energy can cross the membrane faster, whereas particles with less energy travel slower. - Concentration gradient: The concentration gradient across a biological membrane drives the diffusion of a nonelectrolyte. When the concentration differential across the membrane increases, so does the rate of diffusion. As the distribution of molecules across the membrane becomes more uniform, the rate of diffusion drops. Diffusion stops after equilibrium is achieved throughout the membrane.
- Surface area and thickness of biological membranes: The rate of diffusion increases as the membrane’s surface area increases. Increased surface area enhances permeability or mobility of molecules, which is one of the elements responsible for flow.
Similarly, the rate of diffusion decreases as the membrane thickness increases. - Solubility: The solubility of molecules in a medium influences the pace at which particles diffuse.
Lipid-soluble molecules can travel fast across lipid layers, such as the plasma membrane.
Similarly, polar and non-polar molecules flow at various rates depending on the structure of the biological membrane. - Solvent Density: As the solvent density increases, the rate of diffusion drops. The more thick the solvent, the more difficult it is for the solute to move. Solvent density is critical in the transport of solutes in the cytoplasm of the cell. Increased cytoplasm density slows the passage of molecules and gasses, while less dense cytoplasm does the opposite.
Importance of Diffusion
- Diffusion facilitates gaseous exchange during respiration, as well as the removal of carbon dioxide and the intake of oxygen.
- Diffusion aids in the acquisition of gases during photosynthesis in plants. During photosynthesis, it aids in the absorption of carbon dioxide through the stomata of leaves along the gradient.
- Water loss by leaves occurs as a result of diffusion, and this process of water loss in the form of water vapor is known as transpiration.
- Diffusion also facilitates the distribution of chemicals such as ions throughout the cell’s protoplasm.
Example of Diffusion
- When sugar is added to water, the sugar particles travel from a high concentration area (the sugar crystal) to a low concentration area (the surrounding water), causing the solutes to mix gradually.
- When humans breathe, oxygen and carbon dioxide molecules migrate from high concentrations (in the air or blood) to low concentrations (in the lungs or blood), allowing gas exchange to take place.
- When a perfume is sprayed, random thermal motion causes the particles to go from an area of high concentration (close to the source) to an area of low concentration (far away).
- Nutrients, waste products, and other molecules enter and exit cells via diffusion, which can occur across the plasma membrane in response to concentration gradients.
Difference Between Osmosis and Diffusion
Here are some importance difference between osmosis and diffusion:
| Osmosis | Diffusion | |
| Definition | Osmosis is the cell movement wherein in the presence of a semipermeable membrane, the particles move from the areas of low density to the ones with higher concentrations. | The process of diffusion refers to the cell movement wherein the particles move from the areas of higher concentration to the areas of lower concentration |
| Mechanism | It acts on the basis of spontaneous thermal movement of molecules. | Differences in solute concentration drive this phenomenon. |
| Direction | It is unidirectional. Osmosis takes place in one direction. | Multidirectional (molecules travel in all directions until equilibrium is achieved) |
| Driving Force | Osmotic Pressure | Concentration gradient |
| Membrane | This occurs across a selectively permeable membrane. | This can occur across any membrane. |
| Rate of Process | Osmosis is slower than diffusion. | Diffusion is faster than osmosis |
| Medium | Osmosis can only occur in a liquid medium. | Diffusion can occur in all mediums, including solid, liquid, and gas. |
| Types of Solution | This occurs in heterogeneous fluids with a selectively permeable membrane. | Occurs in both homogeneous and heterogeneous solutions. |
| Regulation | Controlled by the differential in solute concentration across the membrane. | It can be influenced by external influences (such as temperature). |
| Control of the process | Osmosis can be stopped and even reversed by applying pressure equal to or greater than the osmotic pressure. | Pressure cannot stop or reverse diffusion. |
| Example | Absorption of water by plant roots | Exchange of oxygen and carbon dioxide in the lungs. |
Similarities Between Diffusion and Osmosis.
Diffusion and osmosis have significant similarities because both involve the movement of molecules. Here are some of the similarities between diffusion and osmosis:
- Passive processes: Diffusion and osmosis are passive processes, which means they do not require energy from the cell or organism. The concentration gradient causes spontaneous movement.
- Moving from high to low concentration: Diffusion and osmosis both migrate from a high concentration location to a low concentration area. This movement continues until equilibrium is achieved.
- Effect of concentration gradient: The concentration gradient influences the rate of diffusion and osmosis, with faster movement occurring when the concentration difference is greater.
- Aims to achieve equilibrium: Both processes ultimately seek equilibrium, which occurs when the concentration of solutes or particles on both sides of the membrane is equal. Once equilibrium is achieved, there is no net movement of molecules.
- Affected by temperature: Both processes are affected by temperature. In general, increasing the temperature causes a faster rate of diffusion and osmosis because it increases the molecules’ kinetic energy.
- Important in biological systems: Both diffusion and osmosis are important processes in biological systems. They perform critical functions in nutrition intake, waste disposal, and cell and organismal homeostasis.
While diffusion encompasses the movement of any sort of particle, osmosis is the flow of water molecules across a selectively permeable membrane. Despite their diversity, these activities share fundamental principles and are essential to the proper functioning of living organisms.
Difference Between Osmosis and Diffusion
References
- https://microbenotes.com/osmosis-and-diffusion/#variation-types-of-osmosis
- https://infinitylearn.com/surge/biology/difference-between-diffusion-and-osmosis/
- https://testbook.com/key-differences/difference-between-diffusion-and-osmosis
- https://www.geeksforgeeks.org/difference-between-osmosis-and-diffusion/
- Helmenstine, Anne Marie, Ph.D. “Differences Between Osmosis and Diffusion.” ThoughtCo, Aug. 25, 2020, thoughtco.com/difference-between-osmosis-and-diffusion-609191.

