Einsteinium is a synthetic chemical element with an atomic number of 99 and is represented by the symbol ‘Es’ in the periodic table. It is silvery in appearance and belongs to the f-block of period 7 of the periodic table. Einsteinium was identified as the seventh synthetic trans-uranium element in the actinide series. Similar to other elements in the actinide class, einsteinium exhibits significant radioactivity.
It is an explosive element found in almost all current nuclear weapons. Albert Ghiorso of the University of California, Berkeley, and another team led by Gregory Robert Choppin at Los Alamos made the initial identification of it in December 1952. They were both looking at the remnants of a nuclear weapon test conducted in November 1952.
Interesting Science Videos
Discovery of Einsteinium
- Einsteinium and fermium were discovered accidentally in waste from the first big hydrogen bomb test, which occurred in the Pacific on October 31, 1952.
- In 1952, scientists at the Los Alamos Scientific Laboratory, Argonne National Laboratory, as well as Lawrence Berkeley National Laboratory synthesized Einsteinium-253 (half-life 20.47 days). Albert Ghiorso oversaw the project.
- The nuclear explosion formed the new element in trace amounts by adding 15 neutrons to uranium-238 (which then went through seven beta decays).
- In 1961, enough einsteinium was created to isolate a macroscopic amount of isotope 253. The sample weighed around 0.01 mg and was measured with a special balance.
- The pure metal was first extracted in the 1970s.
- This element gets its name from Albert Einstein.
Occurrence of Einsteinium
- Einsteinium is a synthetic element that most likely does not occur naturally. Assuming primordial einsteinium originated (from the formation of the Earth), it would have decayed long ago.
- Natural transformation of existing actinides in the earth’s crust into einsteinium is an extremely rare process because it requires multiple neutron capture.
- Multiple neutron capture events from uranium and thorium might possibly yield natural einsteinium however, it is just a theory for now.
- Currently, the element is exclusively created in nuclear reactors or during nuclear weapon tests. It’s created by attacking other actinides with neutrons.
- The High Flux Isotope Reactor (HFIR) at Oak Ridge National Laboratory can yield up to 2 mg of pure element 99.
- Einsteinium has 167isotopes with documented half-lives, ranging in mass from 241 to 257.
Elemental Properties of Einsteinium
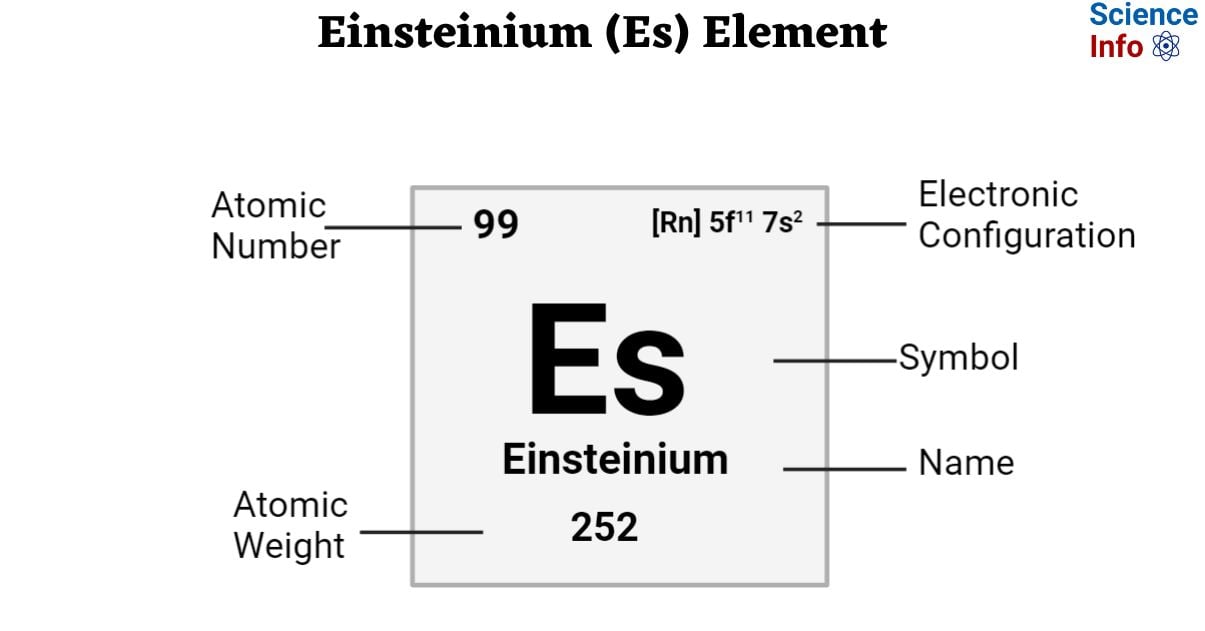
| Electronic Configuration | [Rn] 5f11 7s2 |
| Atomic Number | 99 |
| Atomic Weight | 252 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Actinides, 7, f-block |
| Density | 8.84 g/cm3 at 20 °C |
| Ionic radius | unknown |
| Van der Waals radius | – |
| Electron shells | 2, 8, 18, 32, 29, 8, 2 |
| Electrons | 99 |
| Protons | 99 |
| Neutrons | 153 (Varies with isotopes) |
Isotopic Information of Einsteinium
- All Einsteinium isotopes have radioactive properties.
- Isotopes of element 99 can be created by irradiating lower-atomic-number elements, such as plutonium, with slow neutrons.
- There are 17 known Einsteinium isotopes ranging in mass from 241 to 257 and three identified isomers.
- The longest-living isotopes are 252-Es (471.7 days), 254-Es (257.7 days), and 255-Es (39.8 days).
Physical Properties of Einsteinium
- Einsteinium has physical properties that are similar to fermium and holmium.
- Soft and silvery in appearance, it is metallic.
- It is highly radioactive and has a visible glow, making it appear blue in the dark.
- Einsteinium’s melting point is 860 degrees centigrade. Einsteinium has atomic number 99 and is denoted by the symbol Es.
- It has a somewhat low density of 8.84 g/cm3.
- Einsteinium exhibits Curie-Weiss paramagnetic properties.
- Einsteinium-253, its isotope, emits approximately 1,000 watts of thermal energy per gram.
- Einsteinium crystalizes in face-centered cubic symmetry.
- It possesses a crystalline metal lattice that is damaged by radioactivity and its heat emission.
- Einsteinium has one of the lowest bulk modulus coefficients found in a non-alkali metal.
Chemical Properties of Einsteinium
- It has chemical characteristics similar to those of its close neighbors, fermium and holmium.
- Einsteinium is a reactive element, just like the other actinides.
- Einsteinium compounds with an oxidation state of +3 are relatively stable in aqueous and solid solutions.
- In contrast to many other actinides in solid phase, Einsteinium is divalent.
- Einsteinium compounds with an oxidation state of +4 are hypothesized but have yet to be detected or proved in practice.
- It reacts with oxygen, steam, and acids, but not with alkalis.
- When exposed to water, einsteinium(III) oxide (Es2O3) is generated.
Production of Einsteinium
- Einsteinium-248 is created by bombarding californium-249 with deuterons.
- Einsteinium-249, einsteinium-250, einsteinium-251, and einsteinium-252 are formed by bombarding berkelium-249 with alpha particles.
- When californium-253 beta decays, it produces Einsteinium-253.
Uses of Einsteinium
- Einsteinium currently has no known applications other than basic scientific research into the creation of trans-actinides and higher transuranic elements. It is created in minute quantities as needed.
- Because of its high mass, it is ideal for the manufacturing of ultra-heavy components.
- Einsteinium has only ever been manufactured in trace amounts and is mostly used in scientific study.
- Einsteinium can be used to detect radioactive decay.
- It is one of the most substantial aspects on which bulk investigations can be conducted.
- It has a few medical applications, but none are commercial.
- Its primary application is to study radiation damage, targeted radiation medical interventions, and accelerated aging.
Health Effects of Einsteinium
- Einsteinium doesn’t exist naturally and has not been discovered in the earth’s crust, so there is no reason to worry about its health effects. However, it is extremely harmful due to the radiation it produces.
- Because of its radioactive nature it can be absorbed if consumed, eventually settling in bones, lungs, liver, and testicles where it decays into other, potentially more hazardous radioactive elements.
Environmental Effects of Einsteinium
- Einsteinium doesn’t exist naturally and has not been identified in the earth’s crust, hence there is no reason to be worried about its environmental concerns.
Video on Einsteinium
References
- https://escholarship.org/content/qt8gx712r6/qt8gx712r6.pdf
- https://www.nature.com/articles/nchem.2676.pdf
- https://www.raci.org.au/common/Uploaded%20files/Periodic%20files/422.pdf
- https://link.springer.com/chapter/10.1007/1-4020-3598-5_12
- https://www.thoughtco.com/einsteinium-facts-element-99-or-es-4126476
- https://www.rsc.org/periodic-table/element/99/einsteinium
- https://www.lenntech.com/periodic/elements/es.htm
- https://chemicalengineeringworld.com/einsteinium-element-properties-and-information/
- https://www.chemicool.com/elements/einsteinium.html

