Fermium is a synthetic chemical element with an atomic number of 100 and is represented by the symbol ‘Fm’ in the periodic table. It is silvery in appearance and belongs to the f-block of period 7 of the periodic table. Fermium was identified as the eighth synthetic trans-uranium element in the actinide series. Similar to other elements in the actinide class, fermium exhibits significant radioactivity. It was discovered in 1952 by a group of scientists led by Albert Ghiorso. They discovered fermium-255 in the rubble of the first hydrogen bomb detonation during Operation Ivy. Fermium-255 was formed when uranium-238 reacted with 17 neutrons due to the explosion’s high temperature and pressure.
Interesting Science Videos
Discovery of Fermium
- The initially identified isotope of fermium (later known as fermium-255) was discovered in the nuclear debris following the thermonuclear explosion of the first hydrogen bomb (Ivy Mike) on an archipelago in the Pacific Ocean.
- It was formed when uranium-238, the source of heat for the detonation, was subjected to a flow of neutrons during the process and mixed with 17 of them to produce Fm-255, which has a half-life of roughly 20 hours.
- Fermium was discovered in 1952 when Gregory Choppin, Stanley Thompson, Albert Ghiorso, and Bernard Harvey were investigating post-testing debris. The news of the discovery was kept secret until 1955.
- Later on researchers from the Nobel Institute of Physics in Stockholm were able to synthesize the fermium-250 by bombarding uranium-238 with oxygen-16 ions in 1954.
- It was named after one of the world’s greatest nuclear physicists, Enrico Fermi.
Occurrence of Fermium
- Fermium did occur naturally, along with other transuranic elements, at Oklo’s natural nuclear fission reactor, but this is no longer relevant.
- The natural transition of actinides in the earth’s crust to fermium is an extremely improbable event because it requires numerous neutron captures.
- Fermium is artificially created and is produced in nuclear weapon tests, high-power nuclear reactors, or laboratories, where it remains for only a few days due to its short half life.
- It is synthesized in extremely small quantities by the neutron bombardment of lighter actinides in high flux nuclear reactors. It is the final element that can be created in macroscopic quantities since it is the heaviest element that can be produced through neutron bombardment of lighter elements.
- Due to its exceptionally unstable nature, any fermium created during the formation of the earth would have decayed by this time.
Elemental Properties of Fermium
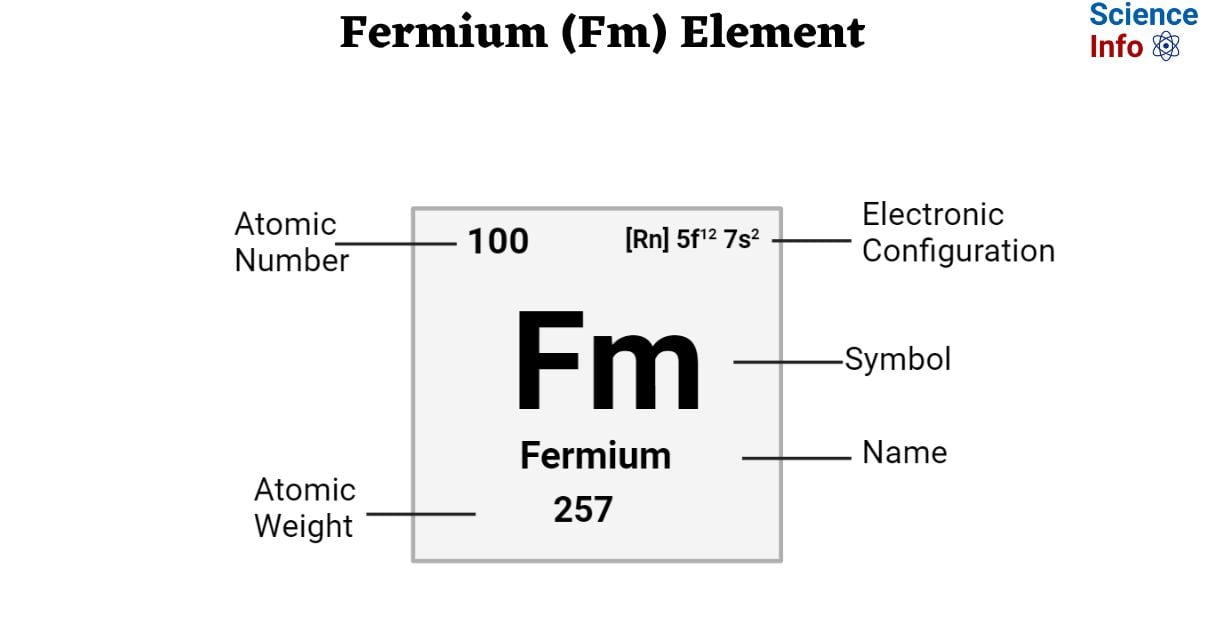
| Electronic Configuration | [Rn] 5f12 7s2 |
| Atomic Number | 100 |
| Atomic Weight | 257 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Actinides, 7, f-block |
| Density | 9.70 g/cm3 at 20 °C |
| Ionic radius | unknown |
| Van der Waals radius | – |
| Electron shells | 2, 8, 18, 32, 30, 8, 2 |
| Electrons | 100 |
| Protons | 100 |
| Neutrons | 157 (Varies with isotopes) |
Isotopic Information of Fermium
- Fermium contains 21 isotopes with confirmed half-lives, ranging in mass from 242 to 259. It does not have naturally occurring isotopes.
- The longest-lived isotopes are 257-Fm (with a half-life of 100.5 days), 253-Fm (with a half-life of 3 days), and 252-Fm (with a half-life of 25.39 hours).
- All of the remaining isotopes have half-lives ranging from several hours to less than a millisecond.
- Fm-250, which has a half-life of 30 minutes, was discovered to be a decay product of nobelium.
- Heavier nuclides are unstable and experience uncontrolled fission, making nuclides with mass numbers more than 257 difficult to synthesize through neutron capture and can only be generated through nuclear explosions.
Physical Properties of Fermium
- The atomic mass of fermium is 257.
- Fermium is highly radioactive element and silvery in appearance.
- Fermium is the heaviest synthetic element that can be created through neutron bombardment of lighter elements, and thus the heaviest element that can be manufactured in macroscopic amounts.
- It has the density of 9.70 g/cm3 at 20 °C.
- The melting point of fermium is predicted to be 1527 °C however the boiling point of the metal is not known yet.
- Fermium in its purest from has not been procured yet.
Chemical Properties of Fermium
- Fermium’s chemical characteristics are unknown due to its synthetic nature. However, it is anticipated to have chemical properties like other elements in the lanthanide and actinide families.
- Fermium’s chemistry is characteristic of the late actinides, having a dominant +3 oxidation state but also a trend toward an accessible +2 oxidation state.
- Although no solid fermium compound has ever been produced, Fm(III) has been examined using co-crystallization procedures as a trace component in a rare earth matrix with the same charge.
- Fermium precipitates along with rare earth fluorides and hydroxides.
- In aqueous solution, fermium is present as the Fm3+ ion.
- Fm3+ ion forms complexes with a wide range of organic ligands including hard donor atoms such as oxygen, and these combinations are typically more stable compared to those of the lighter actinides.
- Fermium ion is capable of creating anionic complexes with ligands like nitrates or chlorides. These are more stable compared to the ions of einsteinium and californium.
- Fm3+ can be effectively reduced into stable Fm2+ using fairly potent reducing agents like samarium(II) chloride.
Chemical Reactions of Fermium
- Reaction With Water
Since very little fermium is produced, it is yet unclear how fermium behaves in water. It is anticipated, nevertheless, that it may approximate that of the element erbium, which is located in the periodic table above fermium.
- Reaction With Air
Because only a trace of fermium is produced, how it reacts with air remains unknown. However, experts believe that fermium may be susceptible to air.
- Reaction With Halogens
Reactivity of fermium has yet to be discovered. However, it is expected to approximate that of erbium, the element slightly above fermium in the periodic table.
- Reaction With Acids
Because only a little amount of fermium is produced, its reaction with acids is unclear. Scientists believe that fermium may be vulnerable to acids.
- Reaction With Bases
Fermium’s reactivity with bases is unknown due to the production of a small amount of fermium. It is projected that it will be the same as erbium, which is found just above fermium in the periodic table.
Production of Fermium
- Fermium is formed through several neutron captures in lighter elements like uranium and curium, followed by sequential beta decays. The likelihood of such catastrophes rises with increasing neutron flux, and nuclear explosions are the most powerful neutron sources on Earth. Fermium is also created by bombarding lighter actinides with neutrons in nuclear reactors or accelerators. Fermium-257 is the heaviest isotope obtained through neutron capture and can only be manufactured in nanogram quantities. The main source is the 85 MW High Flux Isotope Reactor (HFIR) at Oak Ridge National Laboratory in Tennessee, United States.
Uses of Fermium
- Because fermium is generated in small quantities and all of its isotopes have relatively short half-lives, there are no practical applications other than fundamental scientific research.
- Scientists use fermium in their experiments. Because there are many unknown facts regarding this element, scientists are still conducting experiments to gain a wider understanding of it.
Health Effects of Fermium
- Fermium doesn’t exist naturally and has not been discovered in the earth’s crust, so there is no reason to worry about its health effects. However, it is extremely harmful due to the radiation it produces.
- Because of its radioactive nature it can be absorbed if consumed, eventually settling in bones, lungs, liver, and testicles where it decays into other, potentially more hazardous radioactive elements.
Environmental Effects of Fermium
- Fermium doesn’t exist naturally and has not been identified in the earth’s crust, hence there is no reason to be worried about its environmental concerns.
Video Reference
References
- https://periodic.lanl.gov/100.shtml
- https://infinitylearn.com/surge/chemistry/fermium/
- https://www.lenntech.com/periodic/elements/fm.htm
- https://www.vedantu.com/chemistry/fermium
- https://www.chemicool.com/elements/fermium.html
- https://testbook.com/chemistry/fermium
- https://chemicalengineeringworld.com/fermium-element-properties-and-information/
- https://periodic-table.com/fermium/

