Glycerol is a kind of sugar alcohol. Sugar alcohols are a type of polyol that are white, water-soluble organic molecules with the general chemical formula (CHOH)nH2. It is an organic chemical, also referred to as glycyl alcohol or glycerine. It is a colorless, odorless, sweet-tasting liquid. In low quantities, it is non-toxic and viscous at room temperature.
Interesting Science Videos
What is glycerol?
It is tri-hydric alcohol with the chemical formula C3H8O3. The structural formula of glycerol is generated from propane by substituting one hydrogen atom from each carbon with a -OH group.
K. W. Scheele, a Swedish chemist, discovered glycerol by accident while he had been looking into the similarities between soap and the drying plaster Emplastrum simplex. The salve was produced using lead salts of fatty acids, whereas soap is made using sodium salts of organic acids. During his research with lead monoxide and olive oil, he discovered a water-soluble compound with a sweet taste. This was the first chemical isolation of glycerol and was initially referred to as the “sweet principle of fat.”
Pelouze, a French physicist, discovered the chemical formula for glycerol in 1836. He presented a C3H8O3 empirical formula. The structural formula of C3H5(OH)3 was acknowledged fifty years later, based on the findings of two scientists named Berthelot and Lucea.
It plays a role in both carbohydrate and lipid metabolism. It is also utilized in the pharmaceutical, cosmetic, detergent, and plastics industries, as well as the food sector. It is also used in the manufacture of explosives.
Structure of glycerol
It is a trihydroxy sugar alcohol with three carbon atoms and three hydroxyl groups.
It has the chemical formula C3H8O3 and the extended formula CH2OH-CHOH-CH2OH. Furthermore, glycerol’s IUPAC name is 1, 2, 3- Trihydroxypropane or 1, 2, 3- Propanetriol. It has a molar mass of 92.09 g/mol1. Furthermore, it comprises a three-carbon molecular chain with three hydrogens substituted by three hydroxyl groups (-OH). Because every carbon atom in it has an sp3 shape, the molecule possesses free rotation in all bonds. It is also prochiral.
Occurrence of glycerol
It is a triglyceride found in various plants, including soybean, livestock, and palm. It is also produced in industry through saponification or hydrolysis of triglycerides.
It has been identified as an intermediate in carbohydrate and lipid metabolism in biological systems because excess carbohydrates can be transformed into long-chain fatty acids and esterified with the three hydroxyl groups.
Preparation of glycerol
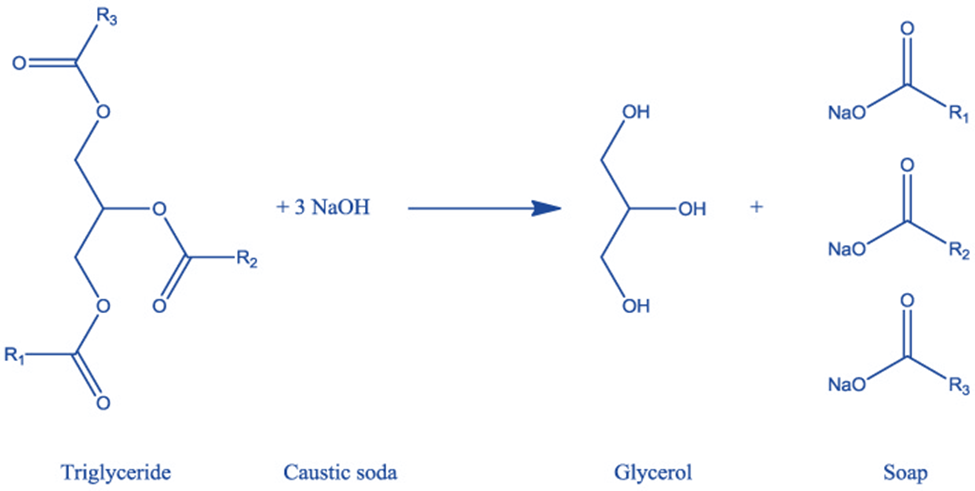
Hydrolysis of oil
This reaction occurs between triglyceride and alkaline hydroxide (caustic soda), resulting in the formation of glycerol and soap.
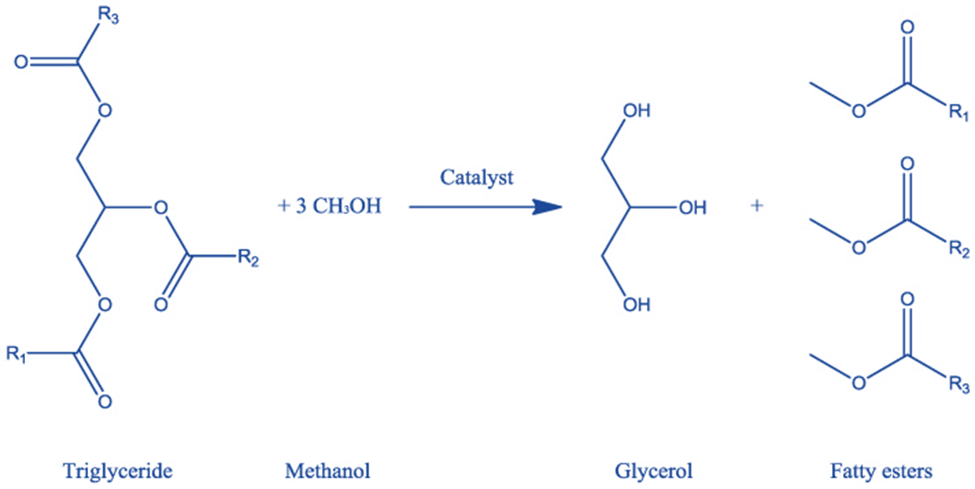
Transesterification of oil
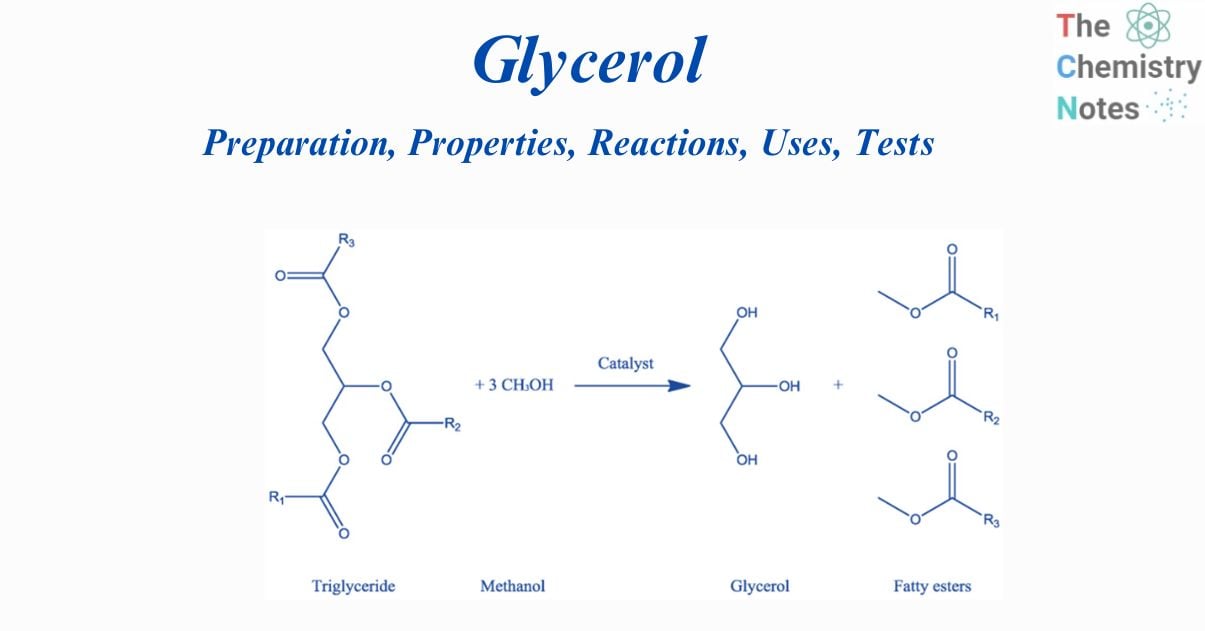
Rochieder conducted the transesterification reaction of beaver oil with ethanol to generate glycerol in 1864. Methyl-esters from triglycerides (oils) and methanol (alcohol) react to form glycerol and fatty esters (or biodiesel) in these processes.
Properties of glycerol
- The melting point of pure glycerol is 17.8°C. It has a boiling point of 290°C but decomposes at that temperature.
- Due to the presence of three hydroxyl groups, the chemical is hygroscopic and has a propensity to collect moisture from the air. As a result, it can be used as a humectant in cosmetics and food, keeping water and preventing the product from drying out.
- Because polyol groups may form hydrogen bonds with water molecules, It is easily soluble in water.
- It has a specific gravity of 1.26, making it somewhat denser than water.
- Because of its hygroscopic nature, it can cause moderate irritation to the eyes, nose, lungs, and skin. When pure It comes into touch with moist tissues, it can cause drying of the skin and other internal organs.
- Its three hydroxyl groups enable interactions with numerous organic acids to produce esters. A triglyceride is generated when all three reactive groups are esterified with long-chain organic fatty acids. Triglycerides are one of the most prevalent fats in the human body.
Chemical properties of glycerol
Reaction with nitric acid
When It is added to a cold mixture of concentrated sulfuric acid and concentrated nitric acid, an explosive chemical known as Glyceryl Trinitrate is formed. It is known as Nitroglycerin. Nitroglycerin is a poisonous, oily liquid. It is a significant explosive used in the production of dynamite. Alfred Nobel made this discovery. It is also known as Noble’s oil. This reaction must be carried out at a temperature of less than 20°C, otherwise, an explosion will occur.
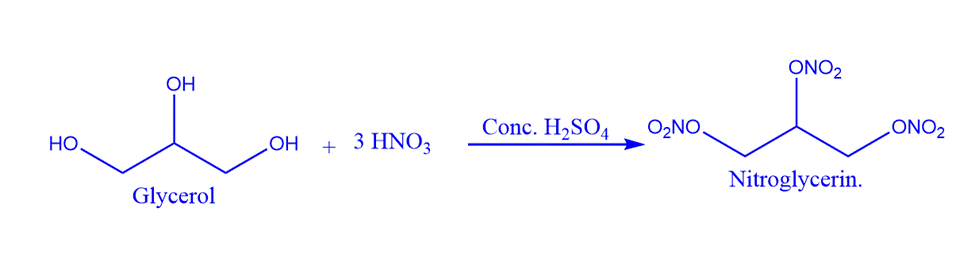
Reaction with oxalic acid
Glycerol and oxalic acid are heated to 260°C to produce allyl alcohol.
CH2OH – CHOH -CH2OH + HOOC – COOH → CH2 = CH – CH2OH
Oxalic acid Allyl alcohol
Reaction with phosphoric acid
Glycerol Mono Phosphate is produced via the interaction of glycerol with phosphorous acid at 105°C.
CH2OH – CHOH -CH2OH + H3PO4 → H2PO4 . CH2 – CHOH -CH2OH
Phosphoric acid Glycerol monophosphate
Dehydration
When It is heated with potassium hydrogen sulfate, it is dehydrated, producing acrolein, a deodorant substance.
CH2OH – CHOH -CH2OH + KHSO4 → CH2 = CH-CHO
Acrolein
Reaction with hydroiodic acid
When allyl iodide is formed, it is reacted with a small amount of HI.
CH2OH – CHOH -CH2OH + HI → CH2I – CHI -CH2I → CH2 = CH – CH2I
hydroiodic acid Allyliodide
Reaction with phosphorus pentachloride:
PCl5 interacts with glycerol and replaces the -OH group with chlorine atoms to give 1,2,3 trichloropropane.
CH2OH – CHOH -CH2OH + PCl5 → CH2Cl – CHCl -CH2Cl + POCl3 + 3 HCl
phosphorus pentachloride 1,2,3 trichloro propane
Uses of glycerol
- It is used in the manufacturing of antifreeze, textiles, and waxes. It is widely used to make resins, paints, and waxes, as well as cleaning and purifying agents for soldering and in the production of various fabrics and cosmetics.
- Many essential chemical molecules, such as formic acid and allyl alcohol, are produced from glycerol.
- It is used in the production of acid-resistant cement.
- It is utilized in protein tonics as glycerophosphate.
- It is used in the cosmetics industry to maintain moisture and improve the texture of lotions and creams. The capacity of glycerol to retain moisture and its emollient qualities make it a desirable constituent in many moisturizing compositions.
- Its capacity to establish inter-molecular hydrogen bonds, particularly with water molecules, makes it useful in food. This raises the water content of preserved food while maintaining shelf life and improving viscosity and texture.
- Triacetin is a glycerol triple ester produced via an esterifying reaction with acetic acid. It is used in the food business as a plasticizing agent to increase the viscosity of a product. It can also be used as a stabilizer for food products that have to be kept for long periods of time.
- In the pharmaceutical sector, It is used to increase smoothness and flavor. It is used in the manufacture of tablets to make them easier to swallow. The coating has the potential to dissolve within the body. It is frequently used in cough lozenges to give them a sweet taste.
Test for glycerol
- The pink color is generated by adding phenolphthalein drops to a 1% solution of Icing. When glycerol is added to this solution, the pink color disappears. When the solution is heated, the pink color reappears; however, On cooling the solution’s pink color reappears.
- When it is heated with potassium hydrogen sulfate, acrolein is generated, which reduces the Fehling solution and ammonium silver nitrate solution.
References
- https://byjus.com/chemistry/glycerol-formula/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5489851/#:~:text=2.-,Hydrolysis%20of%20Oil,12%5D%20(Figure%206).&text=Hydrolysis%20reaction%20for%20glycerol%20production.
- https://www.toppr.com/guides/chemistry-formulas/glycerol-formula/.
- https://www.biologyonline.com/dictionary/glycerol

good chemist