
37.5% concentrated HCL, according to our knowledge, is not pure HCl. So we’ll have to do some arithmetic to dilute it to the proper concentration. There are other methods for calculating, but we will choose the most accurate one, which is the normality calculation for dilution.
The technique of calculation:
Grams of compound required = (N desired) x (equivalent mass) x (volume in liters desired)
Volume of concentrated acid required = (grams of acid needed) / (percent concentration x specific gravity)The concentration of acid or base in a solution is measured using normality (N). Normality and molarity are connected considering that molarity measures the concentration of ions and compounds in the solution, while normality reflects the molar concentration of an acid or basic component.
Simply multiply the molarity by the quantity of hydrogen (H+) in the acid solution or hydroxide ions (OH–) in the base solution to determine the normality.
(HCL has just one hydrogen ion.)
To dilute 0.1 M HCL from a 37.5% concentrated HCL solution, first determine the normality. Simply multiplying 0.1 M by 1 yields 0.1 N.
The equivalent mass is the molar mass divided by the amount of hydrogen ions.
The molar mass of HCL is 36.4611 g/mol.
Since HCL only has one hydrogen ion, the corresponding mass is 36.4611.
The capacity will be 1 liter.
HCL has a specific gravity of 1.189.
Now using the formula;
- Grams of compound needed = (0.1 N) x (36.4611) x (1 Litre) = 3.6461
- Volume of concentrated acid needed = (3.6461) / (0.375 x 1.189) = 8.1774 ml
Therefore, 8.1774 ml of 37.5% concentrated HCL is needed to prepare 0.1 N HCL.
Interesting Science Videos
Procedure for Preparing 0.1 N HCl solution
- Pour 100 mL of distilled water into a 1000 mL volumetric flask.
- Add 8.1744 (about 8.2 mL of 37% concentrated HCl) carefully.
- Pour in 700mL of water.
- Allow the solution to cool to room temperature.
- Add the distilled water to increase the volume up to 1000 mL.
- Thus, the required 0.1 N Hcl is now prepared.
Preparation of 0.1N Na2CO3
Na2CO3 is a solid substance
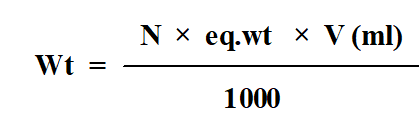
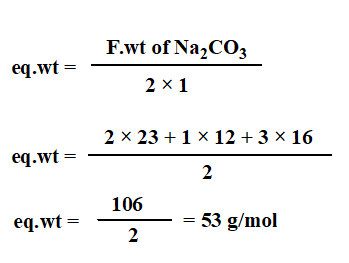
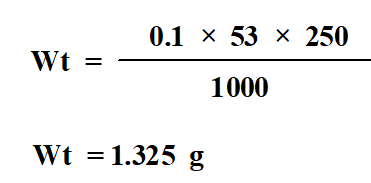
Dissolve 1.325 g Na2CO3 in 250 ml of distilled water to obtain 0.1N Na2CO3.
Standardization of HCl with 0.1 N Sodium Carbonate

Take 2.1 ml of concentrated HCl with a pipet, and dilute to 250 ml with distilled water in a 250ml-volumetric flask to obtain approximately 0.1 N HCl.
Video Reference
References
- https://pharmasciences.in/preparation-and-standardization-of-0-1-n-hcl/
- https://unacademy.com/content/question-answer/chemistry/prepare-a-1n-hci-solution/
- https://www.chemicalforums.com/index.php?topic=1262.0
- https://copharm.uobaghdad.edu.iq/wp-content/uploads/sites/6/uploads/year%202013-2014/chem.lab3rd/2.%20Prep.%20&%20Std.%20of%20HCl.pdf
- https://byjus.com/question-answer/how-can-we-prepare-01-n-hcl-solution/
- https://globalrph.com/dilution/hydrochloric-acid-hcl/
