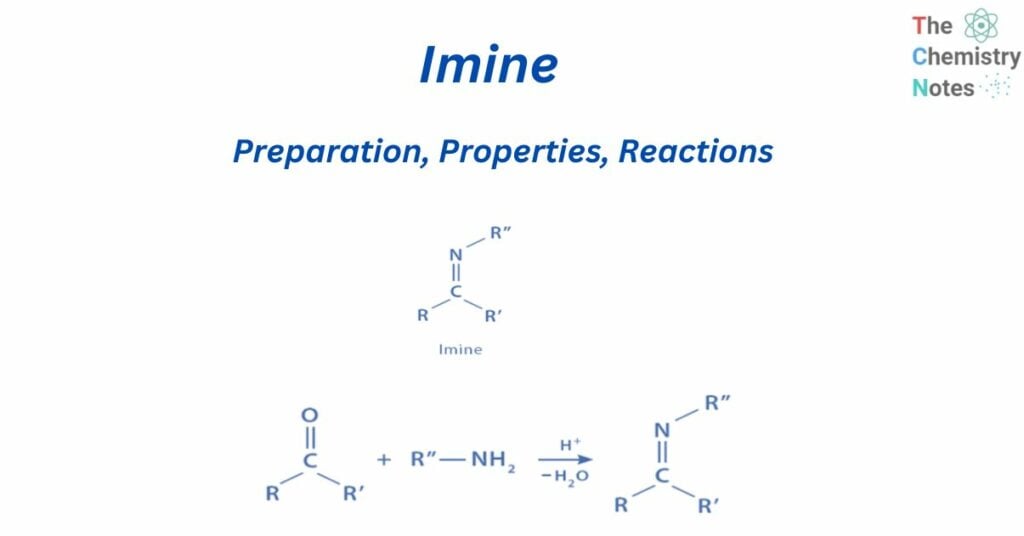
Imine is a chemical molecule with a carbon-nitrogen double bond (C=N). They have a C=N link rather than a C=O bond, and are the nitrogen analogs of aldehydes and ketones. So they are compounds with a carbon-nitrogen double bond – C=N- with substituents at the carbon and nitrogen atoms that can be the same or different.
They are well-known chemicals that are utilized as building blocks in the synthesis of various heterocyclic systems that contain nitrogen and another heteroatom (s) in addition to the nitrogen atom.
Interesting Science Videos
Preparation of Imine
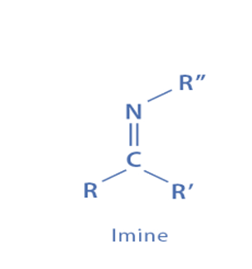
The condensation reaction between an amine and an aldehyde or ketone is one of the most prevalent processes for its production. An acid or base is often used to catalyze this process, which aids in the formation of the imine bond.
They are typically formed as a result of the condensation reaction between a carbonyl molecule and NH3 or an amine. Since amines are stable when the nitrogen substituent is an aromatic ring, they are more stable than compounds formed with NH3.
Although this method of their production is reversible, requiring extensive reaction durations and the use of acid catalysts and dehydration agents such as molecular sieves or azeotropic water removal to bring the reaction to completion, it remains the most frequent way of their preparation. Imines produced from aldehydes and ketones are known as aldimines and ketimines, respectively.
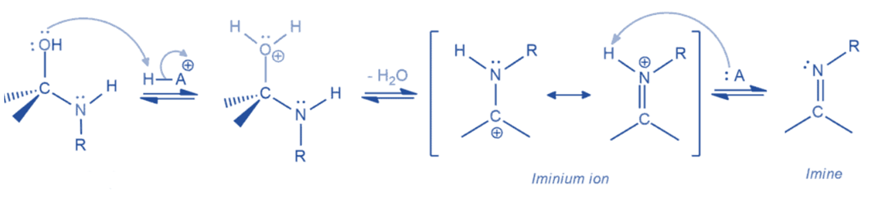
Mechanism
They are formed when an aldehyde or ketone reacts with a primary amine. When aldehydes and ketones are exposed to a primary amine, they first create carbinolamine or hemiaminals, which rapidly lose water to form a carbon-nitrogen double bond. This is known as an imine or Schiff base. Two molecules are united by the removal of water, which is why this process is called condensation.
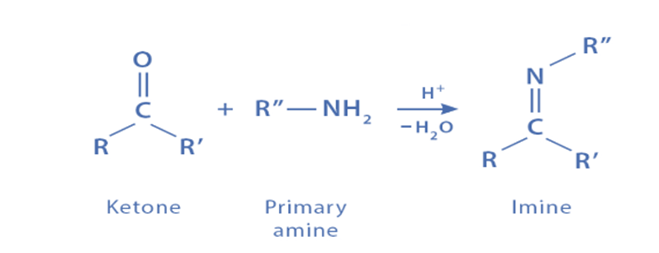
Step I: Formation of carbinolamine or hemiaminal intermediate
As a nucleophile, the amine attacks the electrophilic carbonyl group to create an intermediate. The negative charge produced from this attack is balanced by the protonation of this intermediate. This intermediate is deprotonated to yield hemiaminal or carbinolamine in the final step.
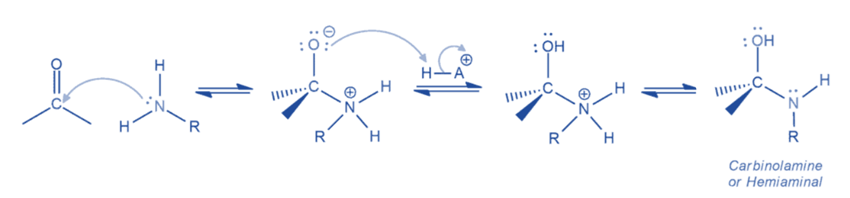
Step II: Conversion of carbinolamine to imine
This is the mechanism of water elimination from carbinolamine that starts with the protonation of the hydroxyl group, transforming it into an excellent leaving group. After the removal of the leaving group, an iminium ion is generated, which is stabilized by resonance. The iminium ion is then deprotonated to produce an imine.
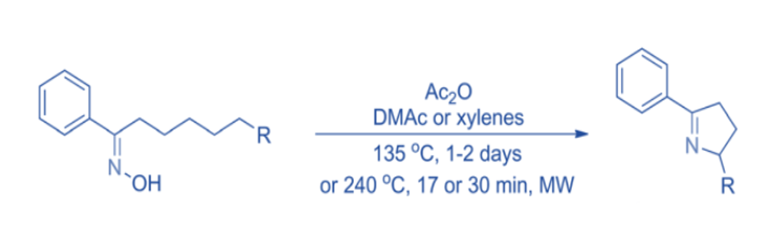
Under suitable conditions, oximes and their derivatives can be cyclized to produce cyclic imines.
Reactions of Imine
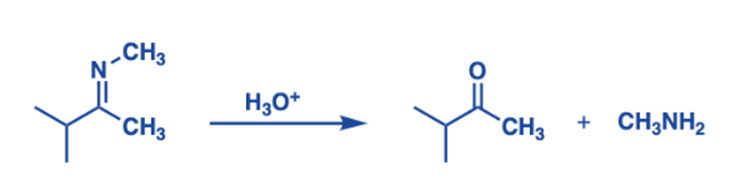
Hydrolysis
Hydrolysis is an important class of its reaction. Hydrolysis processes require cleaving the carbon-nitrogen double bond in an imine by adding water, resulting in an amine and an aldehyde or ketone. A proton (H+) is supplied to the nitrogen atom in their acidic hydrolysis, which is followed by a nucleophilic attack of water on the electrophilic carbon to generate the corresponding amine and carbonyl molecule.
The nitrogen of the C=N bond protonates easily, resulting in a protonated imine. This acts as an electron-accepting group, which can take electrons away from the bonds in the linked molecule when present in an adduct. This facilitates bond cleavage, which is required for numerous metabolic events, such as glycolysis and amino acid metabolism.
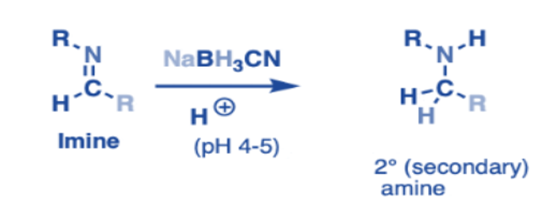
Reduction
They are reduced by adding hydrogen atoms to the carbon-nitrogen double bond (C=N), resulting in the synthesis of primary amines. Reducing agents such as sodium borohydride (NaBH4) or lithium aluminum hydride (LiAlH4) are commonly used in this process.
The reduction pathway begins with the reducing agent attacking the carbon atom of the imine, followed by the transfer of hydride ions to the nitrogen atom. This mechanism eventually changes the C=N bond into a single C-N bond. This reduction reaction is a significant advancement in organic chemistry because it allows for the synthesis of primary amines, which are necessary building blocks for a variety of organic compounds, including medicines and complex organic molecules.
Fischer indole synthesis
The Fischer indole synthesis is the process of producing indoles by converting aryl hydrazones. The significance of chemicals that contain this moiety, including serotonin, tryptamine, and melatonin. Tryptamines are formed when 2-perfluoroalkyl-substituted cyclic imines combine with aryl hydrazines. Because of the increased electrophilicity of the imines bearing -CF3 groups, the reactivity of trifluoromethylated cyclic imines is noticeably higher than that of their nonfluorinated member.
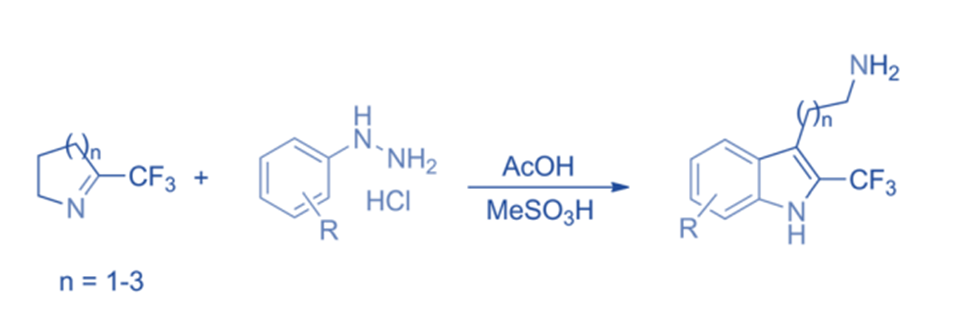
Mannich reaction
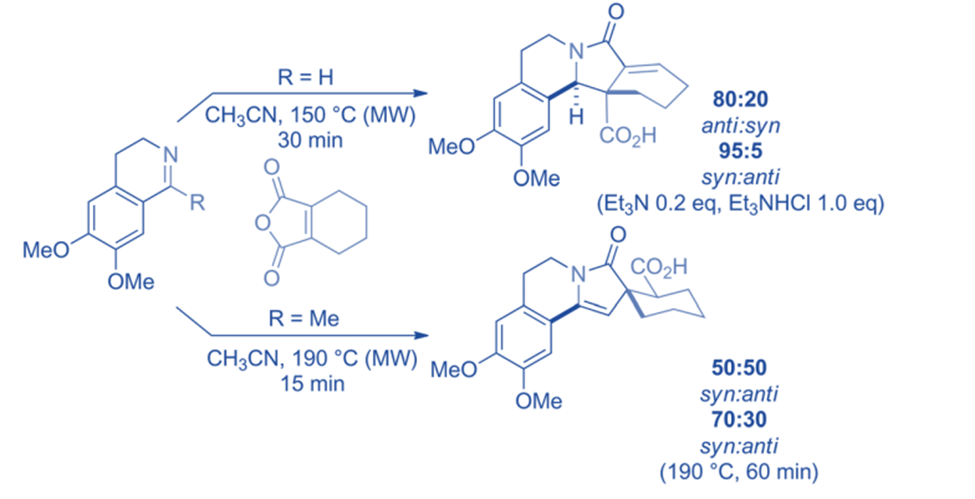
Between cyclic imines and substituted maleic anhydrides, there are Mannich and aza-Michael-like reactions. An aldimine reacted by a Mannich-like mechanism, generating a polycyclic product, while a ketimine created a similar lactam via acylation/aza-Michael reaction. Interestingly, using triethylamine and triethylammonium hydrochloride reversed the diastereoselectivity of the reaction with aldimine. The ketimine was converted into an equimolar mixture of diastereomeric compounds, but after an hour of heating, the composition changed to 70: 30 (syn: anti). The processes can be utilized to make polycyclic lactams and three- to five-membered ring heterocycles, both of which are interesting bioactive molecules.
Imine complexes
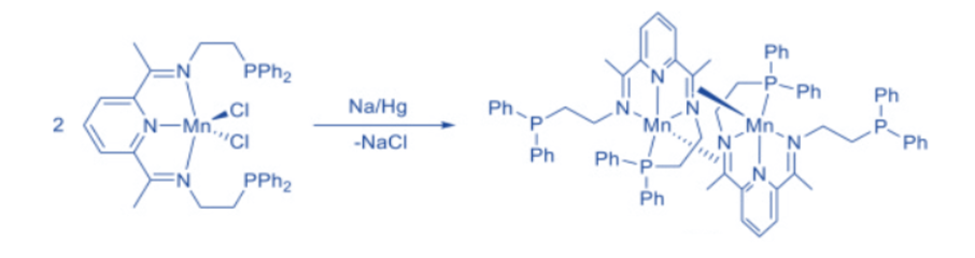
Imine ligands are common in transition metal chemistry and frequently bind to metals in a η1-fashion through the N donor. They, on the other hand, can participate in η2-coordination. A manganese complex with imine ligands that are η 1-N-coordinated to one metal and η2-C, N-coordinated to the other was created by reducing a Mn(II) dichloride complex containing a bis(imine)-pyridine pincer ligand.
Betti reaction
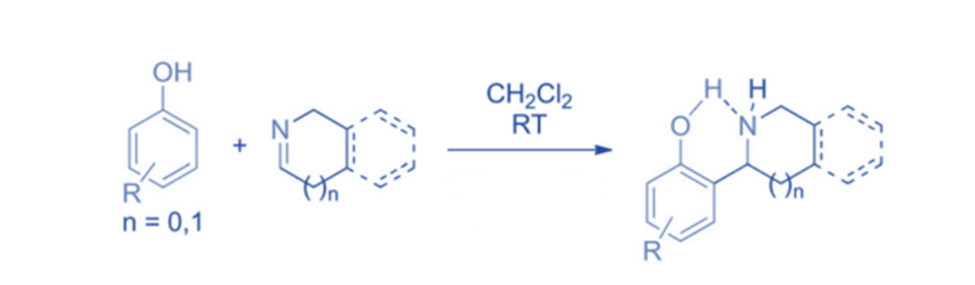
The Betti reaction, named after the Italian scientist Mario Betti, involves the nucleophilic addition of phenolic reagents to imines, leading to the production of amino naphthols and aminophenols. Hydrogen bond creation in the transition state caused the reactions to be very ortho-regioselective. The presence of a hydroxyl group was discovered to be required for the reaction to proceed. When electron-withdrawing substituents in the aromatic ring or methylated imine at position 2 were present, the reaction did not occur.
Henry reaction
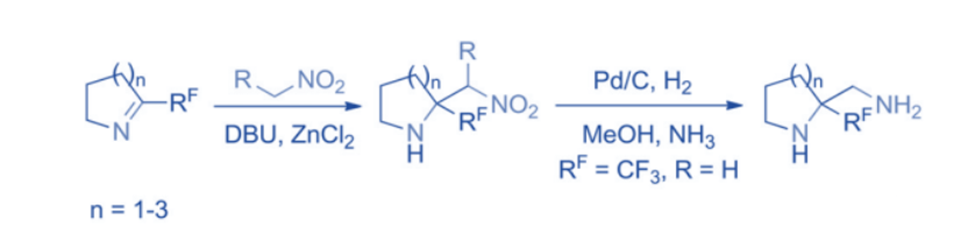
The Henry reaction, as a potent technique for carbon-carbon bond synthesis, produces β-nitro alcohols while opening the way to β-nitroamines when applied to imines (aza-Henry or nitro-Mannich reaction). The aza-Henry reaction of perfluoroalkylated cyclic imines followed by reduction results in the formation of corresponding diamines.
Due to substantial steric hindrance, pentafluoroethylated imines were shown to be much less reactive than their trifluoromethylated counterparts.
Imine Vs enamine
Imine and enamine are nitrogen-containing chemical compounds. Imines are chemical compounds that have a C=N functional group, whereas Enamines contain an amine group that is next to a C=C double bond. The primary difference between imine and enamine is that imine has a C=N bond, whereas enamine has a C-N bond. Imines are produced when a primary amine (or ammonia) interacts with a carbonyl-containing acid (H+, H3O+). It resembles a carbonyl in its most basic form but with a double bond to nitrogen rather than oxygen. When carbonyls combine with a secondary amine, enamines are produced, which have a double bond to carbon rather than nitrogen.
References
- Arun Bahl, and B.S. Bahl (2006). A textbook of organic chemistry Chemistry. New delhi: S. CHAND.
- https://byjus.com/chemistry/imine/.
- https://www.masterorganicchemistry.com/2022/03/07/imine-formation-reactions-mechanisms/.
- https://www.sciencedirect.com/topics/chemistry/imine
- https://www.organic-chemistry.org/synthesis/C2N/imines.shtm
