Iron is the metallic element with the atomic number 26 and is represented by the symbol ‘Fe’ in the periodic table. It is classified as a transition metal and belongs to the d-block of group 8 (VIII) of the periodic table. With almost 5.6% of the earth’s crust and almost the entire earth’s core made of iron, it is one of the cheapest and most abundant metal. Iron metal is a silvery, lustrous metal which has important magnetic properties.
Iron is generally derived from the minerals hematite (Fe2O3) and magnetite (Fe3O4). The minerals taconite, limonite (FeO(OH)n H2O), and siderite (FeCO3) are other important sources.
Interesting Science Videos
History of Iron
- Iron has reportedly been utilized for approximately 5,000 years, according to historians and archaeologists. Iron was occasionally employed earlier in history, but it gained widespread usage during the Iron Age (1200–600 BCE), the third stage of the Stone–Bronze–Iron Age cycle.
- According to certain archeological evidence Iron working may have begun in Zimbabwe and Southeast Africa as early as the 8th century BC.
- It was first practiced in Greece in the late 11th century BC, from where it soon spread throughout Europe.
- Artifacts of smelted iron are found in India dating from 1800 to 1200 BC.
- A stunning iron pillar from Delhi, India, that dates to around the year 400 A.D is still standing today. This solid, wrought-iron pillar is roughly 7.5 meters high and 40 cm in diameter and has not corroded much despite being exposed to different weather conditions since it was installed..
- The first cast iron was created in China in the 5th century BC. Cast iron was employed in ancient Chinese construction, agriculture, and military.
- Due to its lower cost, cast iron started to take over for wrought iron toward the end of the 18th century.
- Iron was getting cheaper and readily available, so it started to become one of the major structural materials used for construction after the first Iron bridge (a cast iron arch bridge that crosses the River Severn in Shropshire, England. ) was built in 1778 which still stands today symbolizing the role of iron in the industrial revolution which laid the foundation of the modern era.
- The name “Iron” is Anglo-Saxon in origin and dates back to the Crusades(1050-1300 CE).
- But its symbol, “Fe,” pays respect to a Latin term that is far older. The term ferrum still inspires modern chemical allusions to element 26 even though it is no longer used to describe iron itself, such as the iron oxide mineral ferrite or the characteristic of ferromagnetism.
Occurrence of Iron
The metal is the fourth most prevalent element in the earth’s crust in terms of weight. In the cosmos, iron is a reasonably common element. The sun and many types of stars contain considerable amounts of it. It has extremely stable nuclei. Iron is a small component of the other two meteorite types but is a major component in a class of meteorites called siderites (FeCO3).
- Several iron ores, including the minerals hematite (Fe2O3), limonite (ferric oxide trihydrate, Fe2O3·3H2O). magnetite(Fe3O4)and taconite(an iron silicate), contain the majority of this iron. The majority of the earth’s core is believed to be made of a metallic iron-nickel alloy.
- The production of iron accounts for nearly 95% of the total tonnage of metals produced worldwide. In an industrial setting, it is created by reducing iron ores with carbon at temperatures around 2,000 °C in a blast furnace.
- With significant amounts also mined in the United States, Canada, Venezuela, Sweden, and India, the primary mining regions are China, Brazil, Australia, Russia, and Ukraine.
Isotopes of Iron
There are four stable isotopes of iron that are found naturally: 54Fe, 56Fe, 57Fe, and 58Fe.
Natural Isotopes Of Iron
| Isotope | Natural abundance (atom %) |
|---|---|
| 54Fe | 5.845 (35) |
| 56Fe | 91.754 (36) |
| 57Fe | 2.119 (10) |
| 58Fe | 0.282 (4) |
- Fe-57 and Fe-58 are the two most often used iron isotopes, and they are mostly used for nutritional studies. Iron loss in human adolescence, circumstances for effective iron absorption, anemia treatments, and genetic iron regulation have all been studied with these isotopes.
Elemental Properties of Iron
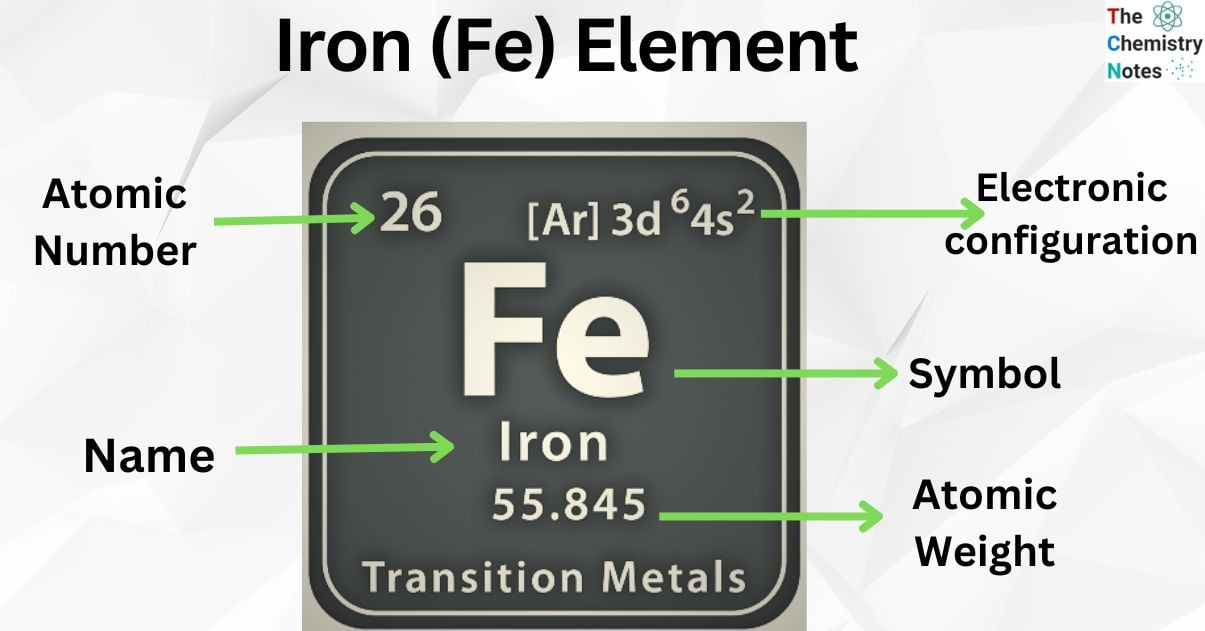
| Electronic Configuration | [Ar] 3d6 4s2 |
| Atomic Number | 26 |
| Atomic Weight | 55.847 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 8, 4, d-block |
| Density | 7.15 g.cm -3 at 20 °C |
| Ionic radius | 0.076 nm (+2) ; 0.064 nm (+3) |
| Van der Waals radius | 0.126 nm |
| Electron shells | 2, 8, 14, 2 |
| Electrons | 26 |
| Protons | 26 |
| Neutrons in most abundant isotope | 30 |
Physical Properties of Iron
- Iron has an atomic number of 26 and is a silvery-gray metal.It has a melting point of 1538 °C (2800 °F) and a boiling point of 2862 °C (5182 °F).
- Iron has a solid phase density of 7.874 gm/cm3 and a liquid or molten phase density of 6.98 gm/cm3.
- It is a recognized as a ferromagnetic element because of its unusual crystalline structure and internal electronic configuration it becomes magnetic once subjected to an external magnetic field.
- Iron is malleable, allowing it to be easily hit into sheets without cleavage, and ductility, which makes it possible to draw thin wires from it.
- Iron has the property of high inherent tensile strength, which makes it easier to shape it. Because of the tensile strength iron has it can provide solidity to structures, which makes it even more popular. Pure iron has a tensile strength of around 7250 psi (pound-force per square inch).
- Iron serves as an excellent electrical conductor. Because electrons in iron are free to move around they are able to carry electrical charge from one end to other.
- Iron is an effective thermal conductor as well. Heat causes a metal’s particles to vibrate more rapidly and move around more swiftly. Energy is transferred from one particle to another as they come into contact.
- Iron’s crystalline structure manifests in three distinct forms, or “allotropes,” rather than in a single form. These three allotropic forms of Iron are known as delta, gamma and alpha Iron. As iron cools from molten form, it takes on these three distinct allotropic forms at different temperatures.
- It starts to rust in humid air and at high temperatures, but not in dry air.
| Color/physical appearance | Lustrous, metallic, greyish tinge |
| Melting point/freezing point | 1538°C, 2800°F, 1811 K |
| Boiling point | 2861°C, 5182°F, 3134 K |
| Density | 7.8 g cm-3 at 20° |
| Malleability | Yes |
| Ductility | Yes |
| Electronegativity | 1.83 (Pauling Scale) 1.8 (Allen Scale) |
Chemical Properties of Iron
- Iron is a highly reactive metal that generates rust, or Fe2O3, when it reacts with oxygen and moisture in the air.
- The oxidation states of the iron span from 2 to +6, however, the +2 and +3, states are the most frequent.
- Iron forms compounds in a wide range of oxidation states, from −2 to +7.
Chemical Reaction of Iron
- The reaction of iron with air
Iron metal oxidizes in humid air, producing hydrated iron oxide as a byproduct. This doesn’t stop the iron surface from reacting further since it flakes off, exposing more iron metal to oxidation. Any car owner is aware with this process, which is known as rusting.Because it is pyrophoric, finely ground iron powder poses a fire hazard.
The iron oxides (Fe2O3) and (Fe3O4) are created when oxygen, O2, is heated.
4Fe(s) + 3O2(g) → 2Fe2O3(s)
3Fe(s) + 2O2(g) → Fe3O4(s)
- Reaction of iron with water
Iron doesn’t react with water under normal circumstances.
- Reaction of iron with the halogens
Iron reacts with excess of the halogens fluorine F2, chlorine Cl2, and bromine, Br2, to form ferric, that is, Fe(III), halides.
2Fe(s) + 3F2(g) → 2FeF3(s) (white)
2Fe(s) + 3Cl2(g) → 2FeCl3(s) (dark brown)
2Fe(s) + 3Br2(l) → 2FeBr3(s) (reddish brown)
This reaction is not very successful for iodine because of thermodynamic problems. The iron(III) is too oxidizing and the iodide is too reducing. The direct reaction between iron metal and iodine can be used to prepare iron (II) iodide, FeI2.
Fe(s) + I2(s) → FeI2(s) (grey)
- Reaction of iron with acids
In the absence of oxygen, iron metal dissolves easily in diluted sulfuric acid to generate solutions that contain the aquated Fe(II) ion and hydrogen gas, H2. Fe(II) is typically found as the complex ion [Fe(OH2)6]2+.
Fe(s) + H2SO4(aq) → Fe2+(aq) + SO42-(aq) + H2(g)
The strongly oxidizing concentrated nitric acid, HNO3, reacts on the surface of iron and passivates the surface.
Uses of Iron
Iron has been used by humans for a very long time of a period. Different artifacts and structures from ancient shows how the use of iron has been done for thousands of year. There are tonnes of uses for iron in the modern era.
Used For Buildings
Due to its low cost and the abundance of iron, it is the most popular metal used in buildings. Metal iron rods are used to construct huge buildings and structures all over the world. They are mostly used for support and are also built into other parts of a building like doors and windows.
Used In Home appliances
Because Iron metal can also be formed into a variety of shapes more readily than many other metals .From spoons to the cooking utensils, from cooking pots to dishwasher, there has been the use of iron. The impact of these appliances on our life is so much that we won’t not be able to perform our daily routines if it wasn’t for iron. Iron is the key element used in the making of different household appliances that are being used by the millions of houses across the globe.
Used In Construction Tools
Construction has gotten much more easier and faster nowadays all thanks to modern tools and equipments. Huge range of tools and equipments are made from iron.
Used For Making Magnets
Iron is used in making magnets. Iron, often known as magnetite, is a naturally occurring permanent magnet. Each atom of neutral iron has four unpaired electrons. Each electron is a very small magnet, but visible magnetic qualities need a large number of such tiny magnets virtually lined up in the same direction.
Used As a Catalyst
Iron is used as a catalyst for the production of ammonia, method is known as the Haber process and involves the combination of hydrogen and nitrogen. Hydrogen is mostly obtained from natural gas, while nitrogen is obtained from the air. Ammonia is important for the production of fertilizer in agriculture.
Health Effects Of Iron
Every day, people require 10 to 18 milligrams of iron. A lot of iron can be found in foods like liver, kidney, molasses, brewer’s yeast, chocolate, and licorice.
The majority of the body’s iron is found in muscle and red blood cells. Meat, fish, beans, spinach, and cereal are examples of food sources with iron.Iron helps red blood cells carry oxygen from the lungs to cells all over the body. Iron also plays a role in many important functions in the body. Iron is frequently used to prevent and cure various types of anemia caused on by low iron levels.
Production Of Hemoglobin
Hemoglobin, a protein found in red blood cells that carries oxygen throughout the body, is made primarily of iron. Additionally, hemoglobin plays a critical role in replenishing blood lost during transfusions, particularly when it comes to women’s menstruation.
Muscle function
Muscular tissues contain a significant amount of iron, especially myoglobin, that helps in supplying the necessary amount of oxygen for muscle contraction. It aids in preserving the flexibility and function of muscles.
Regulation Of Body Temperature
The control of a healthy body temperature is aided by iron. According to the body’s capability for absorption, it can control temperature. A normal temperature promotes an ideal environment for the execution of metabolic and enzymatic processes.
Boosts Immunity
The human immune system depends heavily on iron for its power, which enables it to fight off illnesses and infections. Iron aids in the recovery process by providing damaged cells with the essential oxygen they require.
Treats Anemia
It has been reported to be effective in the treatment of anemia, a serious condition brought on by hemorrhage or an iron shortage. In order to make up for their blood loss after giving birth, women are also given iron supplements.
Insomnia
Iron has been demonstrated to help persons with insomnia sleep better. It assists with controlling circadian cycles. Additionally, it lessens blood pressure fluctuations, which are thought to keep people awake at night.
Iron Toxicity
- Corrosive or cellular iron toxicity is a kind of iron toxicity. The gastrointestinal (GI) mucosa can be severely damaged by ingested iron, which can produce nausea, vomiting, abdominal discomfort, hematemesis, and diarrhea. Patients may also experience hypovolemia due to considerable fluid and blood loss.
- If iron comes into touch with and stays in the tissues, it may result in conjunctivitis, choroiditis, and retinitis. A benign pneumoconiosis known as siderosis may develop as a result of prolonged exposure to high amounts of iron oxide fumes or dust.
- Elemental iron taken orally in doses less than 20 mg/kg is not harmful. When 20 mg/kg to 60 mg/kg are consumed, mild symptoms appear. A dose of over 60 mg/kg can cause severe toxicity, severe morbidity, and serious fatality.
- 10% of the iron that is consumed is absorbed from the intestine and then bound to transferrin. Total iron-binding capacity (TIBC) varies from 300 to 400 micrograms/dL while normal blood iron levels are between 50 and 150 micrograms/dL. After significant ingestion of iron, transferrin reaches saturation. Extra iron will circulate as free iron in the blood, which is hazardous to target tissues directly.
- Some of the complications include: Hepatic necrosis, cardiovascular shock, a myocardial condition, Coma Seizures, Coagulopathy, Esophagitis Anemia, ARDS, intestines forming in a stout manner, Gastric perforation.
Environmental Effects of Iron
- Iron ore mining consumes a lot of energy and results in air pollution from diesel generators, trucks, and other machinery that emits nitrous oxide, carbon dioxide, carbon monoxide, and sulfur dioxide.
- Acid and heavy metals that leak from the mines are also contaminated by the mining of iron ore. Even after mining operations have ceased, acid drainage can persist for thousands of years.
- Pentahydrate of Iron (III)-O-arsenite may be dangerous to the environment; particular care should be taken around plants, air, and water. Since the chemical lingers in the environment, it is strongly advised against letting it in.
Watch out about the most interesting Element, Iron.
References
- Verhoeven, J.D. (1975) Fundamentals of Physical Metallurgy, Wiley, New York
- Greenwood, N. N.; Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 978-0-7506-3365-9.
- Hampel, Clifford A. (1968). The Encyclopedia of the Chemical Elements. Van Nostrand Reinhold. ISBN 978-0-442-15598-8.
- https://www.webelements.com/iron/
- https://pubchem.ncbi.nlm.nih.gov/element/Iron#:~:text=Iron%20is%20a%20chemical%20element,a%20solid%20at%20room%20temperature.
- https://www.britannica.com/science/iron-chemical-element
- https://byjus.com/chemistry/iron/
- https://chemistrytalk.org/iron-element/
- https://www.lenntech.com/periodic/elements/fe.htm
- Singhi SC, Baranwal AK, M J. Acute iron poisoning: clinical picture, intensive care needs and outcome. Indian Pediatr. 2003 Dec;40(12):1177-82.
- Sane MR, Malukani K, Kulkarni R, Varun A. Fatal Iron Toxicity in an Adult: Clinical Profile and Review. Indian J Crit Care Med. 2018 Nov;22(11):801-803
- Baranwal AK, Singhi SC. Acute iron poisoning: management guidelines. Indian Pediatr. 2003 Jun;40(6):534-40.

