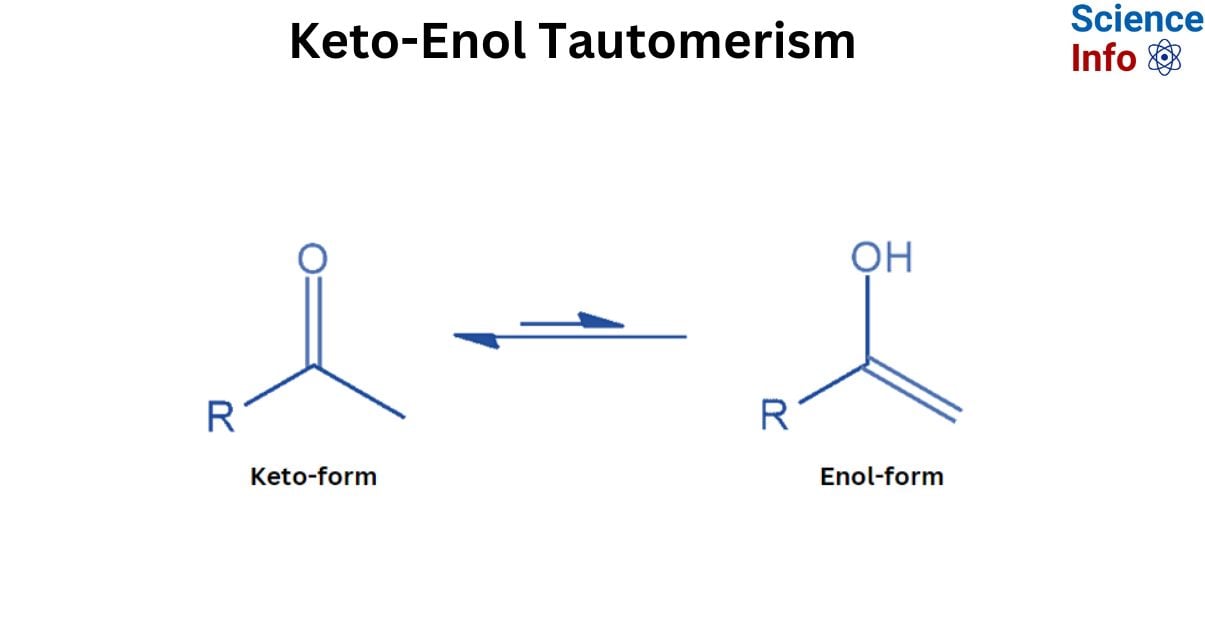
Keto-Enol Tautomerism (KET) is a type of isomerism that occurs between a ketone or aldehyde, known as the ‘keto form’, and an alcohol known as the ‘enol’. The enol and keto versions are considered tautomers of one another.
Tautomers are isomers of a chemical that differ only in their proton and electron positions. The carbon skeleton of the compound remains unaltered. Tautomerism refers to a reaction in which a simple proton transfer occurs via an intramolecular mechanism.
Interesting Science Videos
What is Keto-Enol Tautomerism
In organic chemistry, keto enol tautomerism refers to the chemical equilibrium between a keto form (a ketone or aldehyde) and an enol. Tautomers are believed to occur between the keto and enol forms. The interconversion of the two forms requires the migration of an alpha hydrogen atom as well as the reorganization of bonding electrons; consequently, isomerism is known as tautomerism.
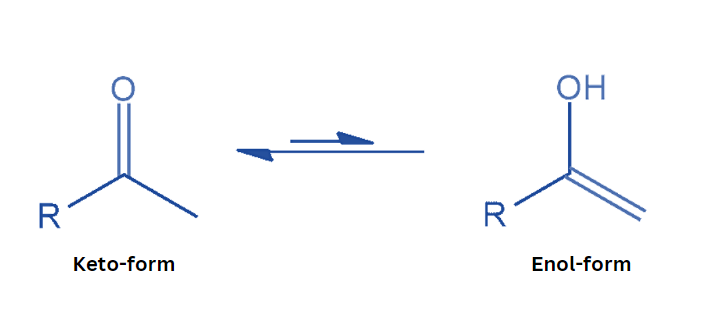
Keto-enol tautomerism occurs when a carbonyl double bond is disrupted and an alkene double bond is created. Keto-enol tautomerism occurs when a compound’s keto form (carrying α-hydrogen) balances with its enol form (a double bond adjacent to an alcohol, -C=C-OH).
Keto-enol Tautomerism Mechanism
Keto enol Tautomerism, a fascinating chemical phenomenon, involves a straightforward two-step process, showcasing the dynamic nature of certain organic compounds.
Acid-catalyzed keto-enol tautomerism
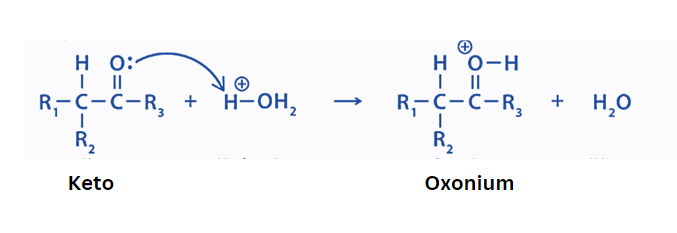
Arthur Lapworth conducted research on acid-catalyzed ketoenol tautomerism in London. In acidic conditions, the keto-enol tautomerism follows the mechanism described below.
Step 1: Carbonyl oxygen absorbs the proton in the first step, resulting in the formation of an oxonium ions.
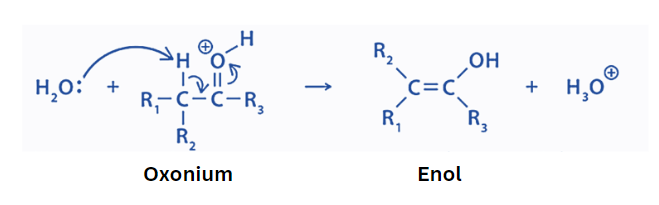
Step 2: In the second step, the base abstracted the alpha hydrogen. This results in the production of enol.
Base-catalyzed keto-enol tautomerism
The mechanism of keto-enol tautomerism in basic conditions is as follows:
Step 1
In the initial step, the base abstracts the alpha hydrogen. This results in the transport of pi electrons towards carbonyl oxygen. This produces an enolate ion.
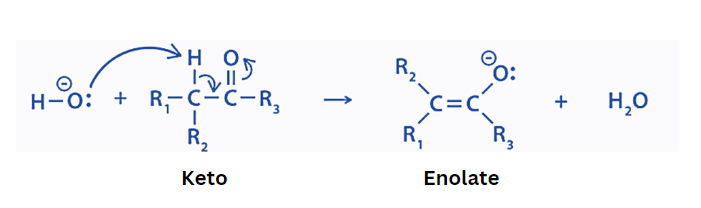
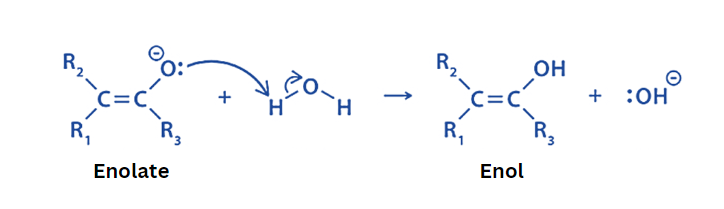
Step 2
In this phase, the oxygen in the enolate ion is protonated to produce enol.
Factors affecting keto-enol tautomerism
There are several elements that influence equilibrium in keto-enol tautomerism.
Stability of keto- and enol forms: The stability of molecules also influences the point of equilibrium. If the keto form is more stable, equilibrium will shift toward it; conversely, if the enol form is more stable, equilibrium will shift toward the latter.
Solvent Factor: Solvent also influences the position of equilibrium in keto-enol tautomerism. Polar protic solvents such as water, alcohol, and acetic acids raise the concentration of the keto form, but non-polar aprotic solvents such as benzene and hexane shift the balance toward the enol form. Similarly, in the absence of solvent, enol content increases.
Steric crowding: Steric crowding has an effect on the equilibrium of keto-enol tautomerism. Steric hindrance reduces the stability of the molecule. The molecules with more steric hindrance are present in small amounts, whereas those with less steric hindrance are present in greater abundance.
Temperature: Temperature has a considerable influence on the position of equilibrium in keto-enol tautomerism. Low temperatures favor the keto form, while high temperatures favor the enol form.
Examples of Keto-Enol Tautomerism
Ketone-Enol Tautomerism in Acetone

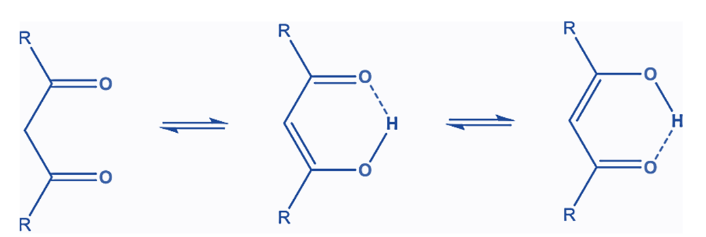
Keto-Enol Tautomerism in Beta-Diketones
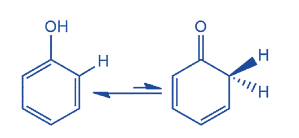
Keto-Enol Tautomerism in Phenol
References
- https://psiberg.com/keto-enol-tautomerism/
- https://www.masterorganicchemistry.com/2022/06/21/keto-enol-tautomerism-key-points/
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_(Morsch_et_al.)/22%3A_Carbonyl_Alpha-Substitution_Reactions/22.01%3A_Keto-Enol_Tautomerism
- https://openstax.org/books/organic-chemistry/pages/22-1-keto-enol-tautomerism
- https://chemistrytalk.org/keto-enol-tautomerization/
- https://www.chemistrylearner.com/keto-enol-tautomerism.html
- https://leah4sci.com/keto-enol-tautomerization-reaction-and-mechanism/
- https://www.britannica.com/science/acid-base-reaction/Acid-base-catalysis#ref498894
- https://www.studysmarter.co.uk/explanations/chemistry/organic-chemistry/keto-enol-tautomerism/
