Molybdenum is a metallic element with the atomic number 42 and is represented by the symbol ‘Mo’ in the periodic table. It is classified as a transition metal and belongs to the d-block of group 6 of the periodic table. Molybdenum does not occur naturally as a free metal on Earth; it only exists in minerals in different stages of oxidation. The free element, a silvery metal with a gray hue, has the sixth-highest melting point of any element.
Molybdenum is the 58th most abundant element in the earth’s crust. Its presence in the crust is about 0.00015% or 1.5 ppm. Its presence in the ocean is estimated to be around 0.001% or 10 ppm, which makes it the 25th most abundant element in the ocean. Powellite (CaMoO4), molybdenite (MoS2), and wulfenite (PbMoO4) are major molybdenum ores.
Interesting Science Videos
History of Molybdenum
- Carl Wilhelm Scheele, a Swedish chemist, discovered molybdenum in 1778 in a mineral known as molybdenite (MoS2), which had previously been wrongly identified as a lead compound.
- In the year 1781, a Swedish chemist, Peter Jacob Hjelm, isolated molybdenum for the first time.
- The name for the element derives from the Greek word “molybdos”,meaning lead.
Occurrence of Molybdenum
- Molybdenum ranks 58th in terms of abundance in the earth’s crust. It exists in the crust at a concentration of around 0.00015%, or 1.5 ppm.
- Its prevalence in the ocean is believed to be roughly 0.001%, or 10 ppm, ranking it as the 25th most abundant element in the ocean.
- It is believed to be the 42nd most abundant element in the universe.
- The major molybdenum ores include Powellite (CaMoO4), molybdenite (MoS2), and wulfenite (PbMoO4).
- Molybdenite ore is the most important source of molybdenum. It is also recovered as a byproduct of the copper and tungsten mining processes.
- The United States, Peru, China, Mexico, and Chile are some of the world’s major producers of molybdenum, among others.
Isotopes of Molybdenum
Molybdenum has seven naturally occurring stable isotopes: 92Mo, 94Mo, 95Mo, 96Mo, 97Mo, 98Mo, and 100Mo.
Naturally occurring isotopes
| Isotopes | Natural abundance (atom %) |
|---|---|
| 92Mo | 14.84 (35) |
| 94Mo | 9.25 (12) |
| 95Mo | 15.92 (13) |
| 96Mo | 16.68 (2) |
| 97Mo | 9.55 (8) |
| 98Mo | 24.13 (31) |
| 100Mo | 9.63 (23) |
Elemental Properties of Molybdenum
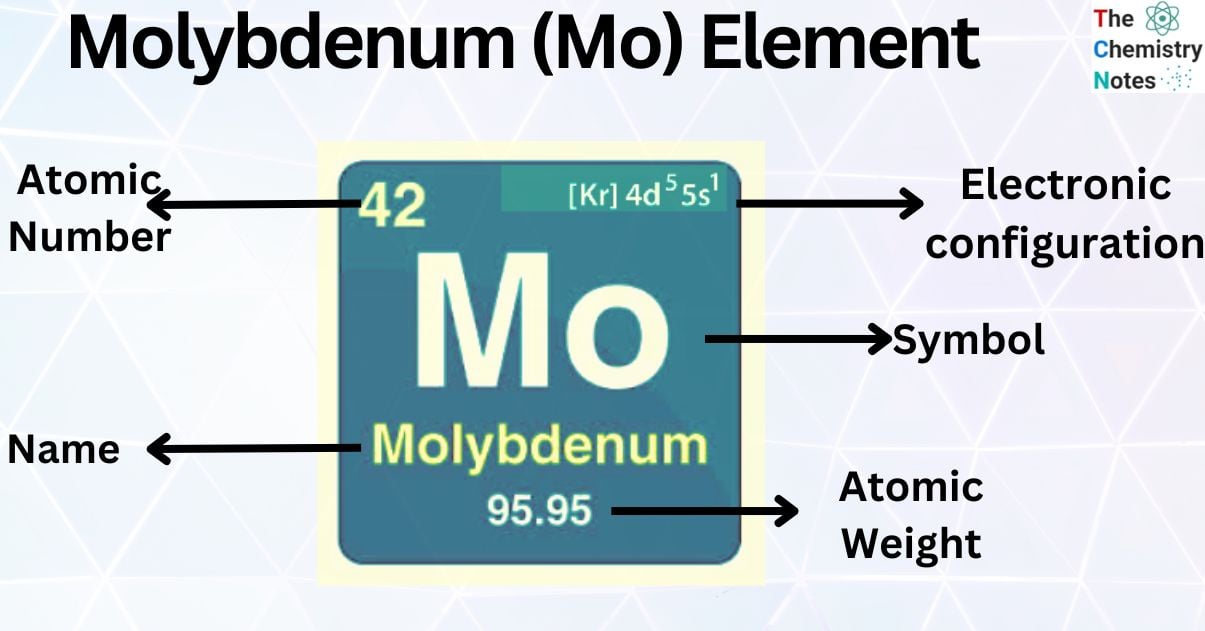
| Electronic Configuration | [Kr] 4d55s1 |
| Atomic Number | 42 |
| Atomic Weight | 95.95 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 6, 5, d-block |
| Density | 10.2 g.cm -3 at 20 °C |
| Ionic radius | 0.068 nm (+4) ; 0.06 nm (+6) |
| Van der Waals radius | 0.139 nm |
| Electron shells | 2,8,18,13,1 |
| Electrons | 42 |
| Protons | 42 |
| Neutrons in most abundant isotope | 56 |
Physical Properties of Molybdenum
- Molybdenum has an atomic number of 42 and is a silvery-white hard transition metal. Only tungsten and tantalum have higher melting points among the easily available metals.
- Molybdenum has a solid phase density of 10.28 grams per cubic centimeter and a liquid or molten phase density of 9.33 grams per cubic centimeter.
- In its elemental form, molybdenum is diamagnetic, which means it repels the magnetic field.
- Molybdenum is brittle in nature; however, if heated above a certain temperature, it loses its brittleness and gains ductility.
- Molybdenum has the property of high inherent tensile strength, which makes it easier to shape it. Because of the tensile strength molybdenum has it can provide solidity to structures, which makes it even more popular.
- Molybdenum has a tensile strength of around 47000 psi (pound-force per square inch) and yield strength of 58000 psi.
- Molybdenum is an effective thermal conductor as well. Heat causes a metal’s particles to vibrate more rapidly and move around more swiftly. Energy is transferred from one particle to another as they come into contact.
- It has a low thermal expansion.
- Molybdenum serves as an excellent electrical conductor. Because electrons in iron are free to move around they are able to carry electrical charge from one end to other.
- It is highly resistant to corrosion.
| Color/physical appearance | Silvery White |
| Melting point/freezing point | 2622°C, 4752°F, 2895 K |
| Boiling point | 4639°C, 8382°F, 4912 K |
| Density | 10.2 g cm-3 at 20° |
Chemical Properties of Molybdenum
- Molybdenum does not react with dry oxygen at ambient temperature, but it rapidly oxidizes above 500 °C or higher and evaporates at 650 °C or higher, forming molybdenum oxide (MoO3).
- The affinity of molybdenum for arsenic is strong.
- It can react with sulfur, carbon, or silicon at higher temperatures to produce Molybdenum disulfide (MoS2), Molybdenum carbide (Mo2C), Molybdenum disilicide (MoSi2).
Chemical Reaction Of Molybdenum
- The Reaction of Molybdenum with Water
At room temperature, molybdenum does not react with water.
- The Reaction Of Molybdenum with Air
Molybdenum does not react with air or oxygen (O2) at room temperature. The trioxide molybdenum (VI) oxide, MoO3, is formed at high temperatures (red heat).
2 Mo (s) + 3 O2 (g) → 2 MoO3 (s)
- The Reaction Of Molybdenum With The Halogens
At room temperature, molybdenum reacts immediately with fluorine, F2, to generate molybdenum (VI) fluoride, MoF6.
Mo (s) + 3 F2 (g) → MoF6 (l) [colourless]
Molybdenum(V) fluoride, MoF5, is generated in the reaction of molybdenum metal and chlorine, Cl2, under precisely regulated conditions.
2 Mo (s) + 5 Cl2 (g) → 2 MoCl5 (s) [black]
Uses of Molybdenum
Molybdenum is a refractory metal with one of the highest melting points of 2620 degrees Celsius among metals. It has a low coefficient of expansion, high conductivity, and good thermal conductivity. Molybdenum does not react with hydrochloric acid, hydrofluoric acid, or alkali solutions at ambient temperature. Therefore, molybdenum and its alloys have numerous applications and potential in the future. Some of these are discussed here:
Used In Electronic Industry
- Molybdenum is an excellent conductor of electricity and can resist higher temperatures. It has a thermal expansion coefficient similar to that of glass, which makes it extremely useful. It is used to produce lead wire, a hook for spiral filament, and the core wire.
Used In Chemical Industry
Molybdenum is utilized in the chemical industry for a variety of applications, including:
As a Catalyst
- Molybdenum compounds are one of the most extensively used catalysts in the chemical, petroleum, textile, and other sectors. The metal is employed as a catalyst in petroleum refineries.
- The process is known as a hydrodesulfurization catalyst, and it is utilized in petroleum refineries to help remove sulfur from natural gas and refined petroleum products.
- Molybdenum can also be used as a catalyst in the manufacturing of polymers and plastics.
Used In Lubricants
- Molybdenum disulfide (MoS2) is an excellent lubricant. This solid lubricant can be utilized routinely under vacuum conditions as well as at varied ultra-low and high temperatures.
- Molybdenum can be mixed with sulfur to make molybdenum disulfide, which aids in the lubrication of two-stroke engines, bicycle coaster brakes, bullets, ski waxes, and other components.
- It is also found in ball and roller-bearing grease employed in the manufacturing, mining, and transportation industries.
Used In Pigments
- Molybdenum is also utilized in paints and dyes as a corrosion-resistant pigment. A typical pigment is made from zinc molybdate (ZnMoO4), which is used in paint primers to avoid corrosion and maintain the color of the paint.
- Molybdate orange (PbCrO4.PbMoO4.PbSO4) pigments are resistant to the effects of fading and corrosion over time. It can be found in paints, inks, polymers, rubber products, and ceramics.
Used As Alloys
- The consumption of molybdenum is highest in the steel industry. The metal helps steel to maintain strength at high temperatures and can endure pressures of up to 300,000 psi.
- Molybdenum also aids in corrosion resistance, making it an important component of stainless steel. Stainless steel alloys containing molybdenum are used in the pharmaceutical industry, chemical mills, and tanker trucks.
- Molybdenum and steel alloy are used to make drills, saws, jet engines, and power-generation turbines. Chrome and molybdenum alloy steel sheets are utilized in mufflers and other automobile components.
- Molybdenum alloyed with cast iron can be used to make cylinder heads, motor blocks, and exhaust manifolds, allowing vehicle engines to run hotter and hence emit less CO2.
Use In Agriculture
- Molybdenum is a trace element that is important for the good health and natural growth of plants.
- Molybdenum helps the plant to absorb phosphorus from the soil. It can also help to increase the chlorophyll and vitamin C in plants. plants can be more resilient to drought, cold climate, and diseases.
Health Effects Of Molybdenum
- Molybdenum is a trace element that is necessary for the human body. A sufficient amount of molybdenum can increase human body growth, improve the storage of oxygen in the body, and prevent tumors.
- Molybdenum is high concentrated in the foods such as grains, legumes, organ meats, etc.
- Molybdenum works as a cofactor to the four different types of enzymes in the human body. These enzymes are responsible for metabolism and breaking down harmful sulfites. without it enzymes would be unable to function, as a result toxin would build up in human body.
- Researches based on molybdenum toxicity on human body are limited. however, the excessive amount of molybdenum may lead to kidney failure, infertility, growth deficiency in animals.
Environmental Effects Of Molybdenum
- While molybdenum is required by both plants and animals, it is hazardous above certain critical amounts. The natural source of molybdenum in the environment is examined. The molybdenum cycle, the relevance of molybdenum in industry and agriculture, and the potential risks that may develop when excessive levels of molybdenum are present in the environment are all discussed.
- Although molybdenum toxicity to humans and non-ruminant animals appears to be low, molybdenum enrichment of the environment from current mine, agricultural, and industrial activities has potentially harmful implications for ruminant animal health.
- Molybdenum occurs naturally in seawater at around 10 ug/l as the molybdate ion, MoO4 2-. Despite high dissolved Mo concentrations in offshore saltwater, phytoplankton from offshore locations contains exceptionally low Mo residues, which is essentially characteristic of Mo-deficient terrestrial plants.
- This occurrence is related to high sulfate concentrations in seawater; sulfate hinders phytoplankton absorption, making molybdate less available in seawater than in freshwater.
- Most livestock would be endangered if fed fodder containing more over 10 ppm molybdenum.
References
- W. M. Haynes, ed., CRC Handbook of Chemistry and Physics, CRC Press/Taylor and Francis, Boca Raton, FL, 95th Edition, Internet Version 2015, accessed December 2014.
- Tables of Physical & Chemical Constants, Kaye & Laby Online, 16th edition, 1995. Version 1.0 (2005), accessed December 2014.
- J. S. Coursey, D. J. Schwab, J. J. Tsai, and R. A. Dragoset, Atomic Weights and Isotopic Compositions (version 4.1), 2015, National Institute of Standards and Technology, Gaithersburg, MD, accessed November 2016.
- T. L. Cottrell, The Strengths of Chemical Bonds, Butterworth, London, 1954.
- chemicool.com/elements/molybdenum.html
- Mary Eagleson, Concise Encyclopedia Chemistry., Walter de Gruyter., 1994., p662.
- https://www.chemistrylearner.com/molybdenum.html
- https://www.rsc.org/periodic-table/element/42/molybdenum
- Mary Elvira Weeks, The Discovery of the Elements V., Journal of Chemical Education., March 1932., p 462.
- https://www.osti.gov/biblio/6782108
