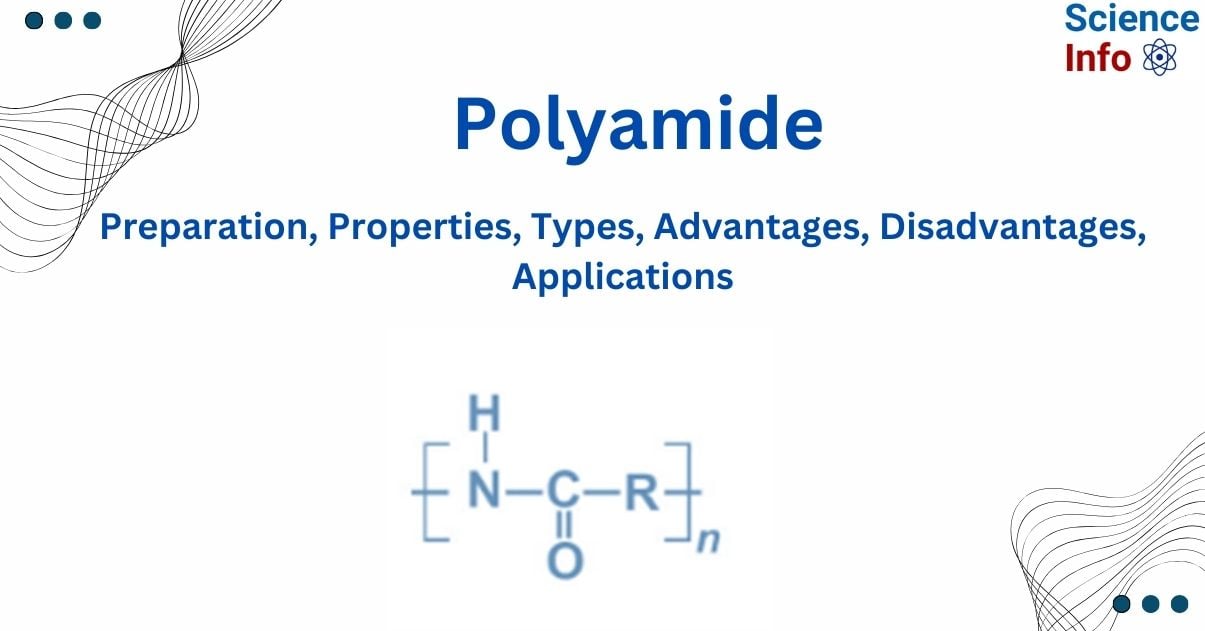
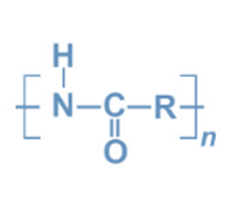
Polyamides are polymers formed by the condensation polymerization of a carboxylic group and an amino group. Polyamides are polymers that contain repeated amide (-CO-NH-) connections. Proteins are naturally occurring polyamides.
During the First World War, an American chemical company named DuPont commercialized polyamide for the first time in 1938. Polyamide is a polymer composed of repeating units held together by amide bonds. They exist naturally, but they can also be synthesized artificially. Proteins such as wool and silk are examples of natural polyamides, whereas synthetic polyamides include nylons, aramids, and sodium poly aspartate.
Polyamides are classified into three families: aliphatic polyamides, semi-aromatic polyamides, and aromatic polyamides (aramids). Nylon resin belongs to the aliphatic polyamides family. The composition and qualities of polyamide differ depending on whether it is aliphatic, aromatic, or semi-aromatic. They are widely recognized for their high strength, durability, chemical resistance, and inexpensive cost. Their great performance and low cost make them useful in a variety of industries, including textiles, engineering, automobiles, electronics, and 3D printing.
Interesting Science Videos
Preparation of polyamide
They occur naturally, however, they are also synthesized using crude oil. The monomer diamine (hexamethylenediamine) is reacted with adipic acid to produce nylon 6,6, the most used polyamide. When these two compounds react, the diamine forms a repeating monomer chain known as a polymer. This newly generated polymer is produced as salt, which is subsequently heated to a molten state. The now molten polymer is extruded via a spinneret, which cools it as it goes through, resulting in a fiber. The fiber is then coiled into a bobbin before being woven into a fabric.
Properties of polyamide
- They have a low density, making it a very lightweight material. They are lightweight and easy to produce, develop, and install.
- Flexural strength is a key property of polyamides. It enables them to bend and adhere to any place. It also improves assembly and installation in areas where rigid metal structures cannot fit. They remain flexible even after prolonged exposure to high temperatures.
- In addition to its flexibility and density, They have the highest tensile strength and impact strength.
- They have a wide temperature range; aliphatic polyamides have a melting temperature of around 500-550K, giving them exceptional heat tolerance.
- The material is exceptionally resistant to wear and abrasion. Its high grade ensures extensive durability in all contact areas.
- It provides successful corrosion against caustic compounds. They are appropriate for harsh environments such as chemical processing plants, refineries, and wastewater treatment plants, where metals corrode after a few years.
- They were specifically designed for high flexibility. This suppleness makes polyamide things easier to move, curve, and fit into tight areas, and it allows for assembly and installation in locations that would not be suitable for rigid metal structures.
Types of polyamide
Aliphatic polyamide
In contrast to aromatic polyamides, aliphatic polyamides lack double or triple carbon-carbon bonds. They have high strength, chemical resistance, flexibility, low creep, and a low coefficient of friction due to their self-lubricating characteristics. They are fairly crystalline when injection molded, although their crystallinity can be increased in fiber applications. They are nylon-class amides.
Nylon 6,6
It has a high melting point and excellent abrasion resistance. As a result, it is chosen for the production of machine parts. It is the most widely used thermoplastic material due to its high heat resistance and strength. It is used to manufacture carpet fibers, electro-insulating elements, bearings, gears, and conveyor belts. It has excellent flexibility and mechanical strength.
Nylon 6
It is a semi-crystalline polyamide with high ductility and abrasion resistance. It is used to create nonwoven fabrics. Nylon 6 was created in an attempt to replicate the qualities of nylon 66 without breaking the patent. It is made using a ring-opening polymerization.
Nylon 6,10
It is utilized as a monofilament. This polymer is used to make filaments for brushes, electric insulators, hosiery, and zip closures. It is resistant to both chemicals and mineral acids. Nylon 6, 10, is a stronger material than most other nylon.
Nylon 11
Nylon 11 is more resistant to dimensional changes due to moisture absorption. This is partially owing to the reduced concentration of amides. It is worth noting that it has less favorable mechanical qualities than other varieties of nylon.
Even though it is made from vegetable oil, it is not biodegradable. It is used to manufacture mechanical components, vehicle fuel lines, sports shoes, tool handles, gears, hydraulic fluid, and powder coatings. It isn’t resistant to concentrated acids or halogens.
Nylon 12
It can be easily formed into any shape or size. This polyamide’s wear resistance is so great that it is unaffected by high-frequency cyclical loads. This nylon compound has the lowest melting point among the primary polyamides. It is commonly used as a flexible film or sheet to protect foods and drugs. It is also relatively resistant to water absorption.
Nylon 4,6
This has the highest fatigue resistance when compared to others. It exhibits great thermal dimensional stability. Nylon 4, 6 has greater chemical resistance and crystallization than nylon 6 and nylon 6, 6. Nylon 46 was created primarily to have a higher operating temperature than other grades of nylon. The advantages of nylon 4,6 include a higher thermal distortion temperature than nylon 6 and nylon 6,6. It also has better crystallinity, which improves chemical resistance, particularly to acidic salts, and reduces cycle times.
Nylon 6,9
It has a lower melting point and absorbs less moisture than other polyamides. It’s used to make electrical connectors, banners, travel bags, furniture, and car upholstery.
Nylon 510
Nylon 510, produced using Penta-methylene diamine (PDA) and Sebacic acid (C10H18O4), was cited in the Carothers patent for nylon 66. It has higher standards of strength and durability but is expensive to manufacture. As it has a higher cost of production its application as fabric is not viable. It is only used for industrial and scientific purposes.
Aromatic polyamide
Aromatic polyamides are also known as aramid or under the trademark Kevlar®. Aromatic polyamides are significantly stronger, more chemically and heat resistant, and have superior dimensional stability than aliphatic polyamides. The chemical makeup of aromatic polyamides is substantially stronger. It is used in hot-air filtration fabrics, optical fiber cables, heat-protective apparel, helmets, jet engine enclosures, loudspeaker diaphragms, and reinforced thermoplastic pipes. Aramids are not conductive but are UV-sensitive. They have excellent resistance to organic solvents and abrasion.
Semi- Aromatic polyamide
Semi-aromatic polyamides are thermoplastic materials with a semi-crystalline structure. The mixture of aliphatic and aromatic polyamides results in a polyamide that is significantly more water resistant and thus more stable. Semi-aromatic polyamides exhibit low creep and strong chemical resistance. They have a mostly crystalline structure.
Advantages of polyamide
- Good thermal stability: They have good thermal stability, which can be utilized at high temperatures.
- Faster to assemble: Their products require less assembly time as compared to their competitor’s materials. They are tiny, lightweight, and compatible with frill-type connectors.
- Ease of Processing: It is easily processed using a variety of processes, including injection molding, extrusion, and stamping.
- High strength: It is a solid synthetic polymer. It has excellent tensile, bending, and stretching strength.
Wear Resistance: It has a strong wear resistance, making it ideal for friction-based applications. - High abrasion resistance: Increased wear resistance owing to mechanical activity.
- Good heat resistance: Certain grades of nylon can melt at temperatures as high as 300°C.
- Good fatigue resistance: This makes it perfect for components in constant cyclic motion, such as gears.
- High machinability: Cast billets can be machined into numerous components that would be too costly to cast into complex shapes.
- Noise Dampening: Nylon is a highly effective noise dampener.
- Multifunctional: They are most suitable for various mechanical qualities and applications. Following the casting process, firms can transform the parts into a variety of multifunctional components.
- Leak-proof seal: Polyamide creates a stiff seal that does not leak. Unlike PVC and other plastics, they do not protect any aromas, so pets will not be misled into chewing through them.
Disadvantages of polyamide
- Water absorption leads to a loss in mechanical characteristics. Nylon 6/12 is specifically designed to prevent moisture absorption.
- UV sensitivity.
- High shrinkage occurs when the product is cooled during manufacturing.
- Electrically insulative.
- Unsustainable.
Applications of polyamide
- It is used to make a variety of textile products, such as garments, shoes, and carpets.
- It is utilized as a coating on several medical appliances. This material’s capacity to endure several chemicals and sterilization makes it an excellent choice for medical equipment. As a result, it is suitable for use in catheters (type of tubes), bandages, walkers, hospital beds, and syringes.
- It is used in the production of a wide range of plastic items, including automobile parts, electrical components, and food containers.
- It is used to manufacture a variety of electronic components, such as connectors, cables, and housings.
- It is commonly used in the automotive industry to make air intake manifolds, engine covers, and valve covers, as well as exterior components such as handles, grilles, wheel covers, and fuel caps and lids. These components were previously made of metal, but polyamide allows for lighter, more fuel-efficient vehicles as well as lowering production costs.
- The food and beverage sector uses polyamide systems to maintain hygiene in places where components may come into contact with food. These systems are widespread in food packaging facilities and manufacturing industries.
- Robotics demands components that are very flexible and resilient, and polyamide offers practical alternatives. It is also handy for protecting wires and cables in robots.
- It is also used as a replacement for various metals. Contrary to metal, nylon is affordable and pliable. As a result, purchasing and producing nylon-based products becomes less expensive. Furthermore, because nylon is more flexible than metal, cleaning instruments made of nylon may reach areas that metal tools cannot effectively clean.
- It is also applicable to some military equipment. Military gear including tents, bags, uniforms, and ropes can benefit from nylon’s strength and resilience to heat and chemicals.
- A polyamide 3D printing filament is a spooled wire that is fed into a 3D printer and then processed into a part. The polyamide filament melts and becomes a liquid, which is then extruded from the printhead and solidified on the print bed.
Comparison between Nylon and Polyamide
The distinctions between polyamide and nylon are due to unique properties within the larger category of synthetic polymers. Their shared amide connections provide remarkable mechanical qualities, making them useful for a wide range of industries. Polyamide and nylon’s extraordinary qualities continue to drive growth and innovation in a variety of sectors, including materials, vehicle parts, plastic design, and athletic gear. As technology advances, we may expect increasingly more advanced standards and applications for these adaptable materials, broadening their reach and impact on modern industries.
Polyamide
Polyamides are polymers made up of repeated units connected by amide linkages. An amide group has the chemical formula CONH2. These repeating components, known as monomers, differ according to the type of polyamide. Polyamides can be both natural and synthetic.
Nylon
Nylon is a type of synthetic polyamide composed of carbon and hydrogen monomers that form chains containing amide groups by a process known as “condensation polymerization. This is a mechanism when water is lost between two monomers. In its synthesis, a dicarboxylic corrosive and a diamine undergo a build-up polymerization reaction. Different varieties of nylon are produced depending on the monomers used and the handling circumstances.
Polyester vs polyamide
Polyester is a synthetic fabric that is mainly manufactured from petroleum. It is a polymer made up of compounds containing the ester functional group. The majority of the fibers are manufactured from ethylene, a component of petroleum. Polyester production might vary depending on the type of polyester manufactured.
There is also plant-based polyester, which uses sugar cane as an ethylene source rather than petroleum. Polyester is significant in the textile business since most cotton-based items, such as coats, suits, and dresses, may also be constructed using polyester.
Polyamide is a polymer composed of strings of polyamide monomers. Polyamides can form naturally or synthetically. Wool and silk are examples of natural polyamides, whereas synthetic polyamides include nylon, aramids, and sodium polyaspartate.
Comparison
- Polyester textiles are known for their strength, which means they are long-lasting and do not wear and break like other fibers. They don’t have a luxurious feel.
Polyamides are typically softer than polyester since they are manufactured as a synthetic replacement for silk. They’re elastic, gentle to the touch but durable, and offer the best abrasion resistance. - Polyester is tough and resilient because of its polymer composition, making it resistant to tear, strain, and pilling.
Polyamides are also extremely strong and chemically resistant, making them ideal for wires and cables. Polyamides are highly flexible and resistant to abrasion, wear, and corrosion. - Polyamides are always thermoplastic, whereas polyester can be thermoplastic or thermoset.
- Polyamide is a more durable substance that effectively blocks UV radiation. In the textile industry, polyamide is utilized for outer clothes, but polyester is lighter, thus it’s used for garments that need to be permeable.
References
- https://www.britannica.com/science/polyamide
- https://www.vedantu.com/chemistry/polyamide
- https://bansaltrading.com/what-is-polyamide-properties-and-uses-of-polyamide
- https://matmatch.com/learn/material/polyamide-nylon
- https://www.essentialchemicalindustry.org/polymers/polyamides.html
- https://greennettletextiles.com/polyester-vs-polyamide/
- https://www.fictiv.com/articles/polyamide-vs-nylon-a-comparison-guide
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4476124/
- https://sintac.es/en/what-is-polyamide/
