Radiopharmaceuticals for Imaging are used for therapeutic purposes in the medical sector. Specifically, radiopharmaceuticals for imaging organ function and disease states are a unique capability of nuclear medicine. Drugs attached to radioactive materials are known as radiopharmaceuticals. They can be employed as therapeutic agents, for diagnostic imaging, or both purposes (theragnostic).

Technetium-99m is the radionuclide that is most frequently employed. Current applications of radio imaging include illness detection, treatment monitoring, and tissue physiology analysis; however, with the emergence of personalized medicine, new applications are being found.
- When a patient receives a radiopharmaceutical (imaging agent), it is continuously observed for diagnostic and therapeutic purposes by a specialized imaging instrument, such as a gamma camera. PET and SPECT imaging are some of these systems.
- Usually, intravenous administration of radiopharmaceuticals is followed by their distribution to a specific organ. The radioactive molecule itself will decide which organ it is sent to, not the radiolabelling.
- A gamma camera, which is a type of scintillation detector, is used to detect γ-radiation externally. The radiation is captured by the camera, creating a two-dimensional image. Another name for this diagnostic procedure is scintigraphy.
- PET, on the other hand, creates a three-dimensional representation of bodily functions. The technique relies on the employment of radionuclides that emit positrons together with the gamma rays that are released as an aftereffect. The so-called tracers, radionuclides, enter the body as components of molecules that are physiologically active.
- PET similarly uses gamma cameras to detect radiation administered inside, but in contemporary scanners, three-dimensional images are frequently obtained using a CT X-ray scan that is carried out concurrently as a component of the same apparatus. A diagnostic X-ray creates a two-dimensional image by sending radiation through the body; on the other hand, scintography relies on the internal build-up of radionuclides.
- Radiopharmaceuticals are used in nuclear medicine for radiation and diagnostic imaging.
- They are vital to medicine overall for helping to diagnose organs and treat pathological disorders, particularly cancer.
- Using their radioactive tracers, radiopharmaceuticals are given orally, intravenously, or by inhalation in the imaging modality to allow viewing of the kidneys, lungs, thyroid, heart, bone metabolism, and blood circulation, among other organs.
- In a therapeutic modality, a high dosage of radiation is administered using certain radiopharmaceuticals that target the sick organ to treat cancer or an overactive thyroid gland.
Interesting Science Videos
Radiopharmaceuticals for Imaging
99mTechnetium
- The atomic number of technetium is 43 and its chemical symbol is Tc. It is the least stable element in terms of isotopes.
- This transition metal has a silvery-grey color. The metastable isomer of 99Tc, a gamma-emitting nuclide frequently employed in diagnostic medicine, is known as technetium-99m. Its brief half-life of about six hours makes it perfect for diagnostic uses—but not for therapeutic ones, as it minimizes the patient’s exposure to radiation.
The safe production and delivery of the products to the clinical context presents a problem when employing radioactive materials. Long half-live radionuclides are typically produced in a nuclear reactor for commercial use and supplied as the final product. Due to their quick disintegration, products containing radionuclides with short half-lives cannot be supplied as the final product. As a result, they are supplied to the clinical environment as long-half-life radionuclides, and at the time of usage, the desired radionuclide is then created and prepared.
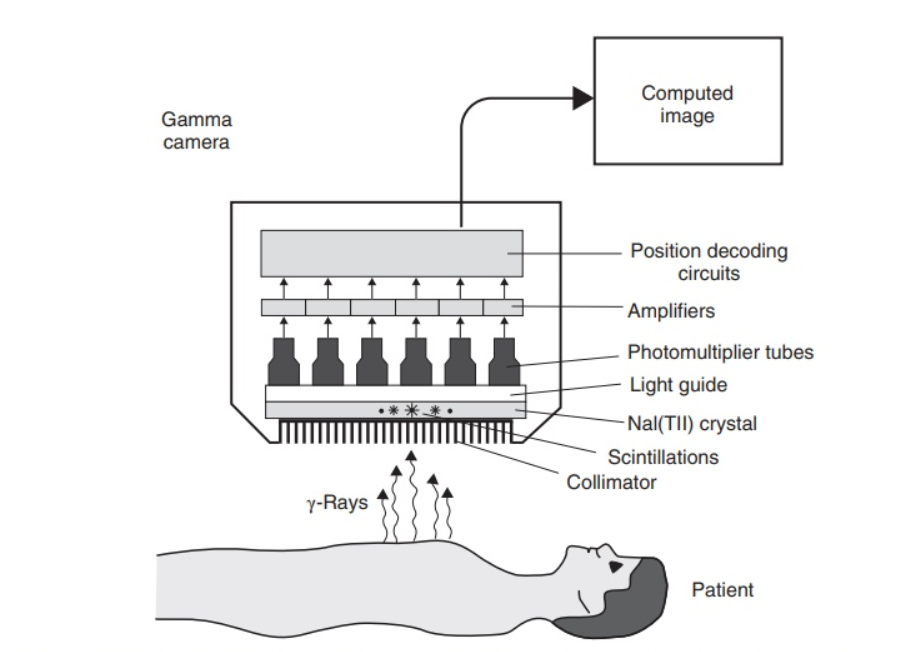
Use of a gamma camera on a patient treated with a 99mTc imaging
agent [Image source: https://www.pharmacy180.com/]
- Using a device known as a “99mTc generator,” 99mTc and its compounds are produced in situ for use as an imaging agent. Molybdenum-99 (99Mo), also known as the transportable source of 99mTc that is commercially accessible, is the fuel that powers the generator. The basic concept is that the generator includes a long-lived “parent” compound that eventually decays to create a radionuclide known as the “daughter.” The 99mTc generator comprises 99mMoO4 2− that has been absorbed on an alumina column. 99mMoO4 2− decays to 99mTcO4 −; this can be eliminated by washing the column with a NaCl solution, which yields Na99mTcO4. Typically, hospitals purchase these generators regularly to guarantee a steady supply of 99mTcO4 −
- 99mTc-containing compounds can be utilized to image a range of bodily structures and activities. Which structure can be observed and to what area of the body the radionuclide is carried depends on the usage of various molecules carrying 99mTc. While 99mTc-albumin is typically used to assess heart activity, many other molecules can be used, such as 99mTc-aerosol, which can be used to image lung ventilation. Human albumin and sodium pertechnetate (NaTcO4) are combined with a reducing agent, such as a tin salt, to create the injectable solution known as 99mTc-albumin.
- Image preparations of the kidney are done with the succimer analog, and skeletal imaging is done with 99mTc-medronate. Sodium pertechnetate (NaTcO4) and meso-2,3-dimercaptosuccinic acid combine in the presence of a reducing agent, such as a stannous salt, to produce 99mTe succimer injection.
- Cardiolite, an organometallic chemical based on 99mTc, has emerged as one of the most used nuclear imaging agents for visualizing anomalies in the parathyroid and heart muscle. 99mTc-sestamibi, a coordination compound of 99mTc with six so-called MIBI ligands, is commercially known as cardiolite. The acronym for methoxyisobutylisonitrile is MIBI. (OC-6-11)-hexakis[1-(isocyano-𝜅C)-2-methoxy-2-methylpropane][99mTc]technetium(I) chloride is the complete chemical name. Tetrakis[(2-methoxy-2-methylpropyl-1-isocyanide)copper(I)] tetrafluoroborate, a weak chelating agent, and sodium pertechnetate (NaTcO4) in the presence of a stannous salt are heated to create a standard injection solution.
18Fluoride: PET scan
- The most electronegative element is fluorine, which has the atomic number nine and the chemical symbol F. It is a member of group 17, or the halogens, in the periodic table.
- At ambient temperature, fluorine normally occurs as a diatomic molecule.
- Of the known fluorine isotopes, only one (19F) is stable. The half-lives of the majority of radioactive isotopes are typically less than one minute. The only radioisotope that is utilized in clinical settings and has a longer half-life—roughly 110 minutes—is 18F.
- PET scanning is one application for 18F, a radioisotope that emits positrons, in radiopharmaceutical imaging.
- Fluorodeoxyglucose (18F-FDG) and its derivatives, which are 18F choline derivatives, are the subjects of extensive clinical research and implementation. A radiolabel (18F) has been added to the 2′ position of the glucose derivative 18F-FDG, replacing the hydroxyl group. Intravenous administration of 18F-FDG is used to evaluate issues with glucose metabolism, particularly in the brain, which is frequently linked to cancer and epilepsy. There is a correlation between locations with enhanced glucose metabolism and those with apparent higher 18F-FDG absorption. Similar to glucose, 18F-FDG is transported throughout the body and eliminated via the kidneys. There are no recognized side effects associated with 18F-FDG.
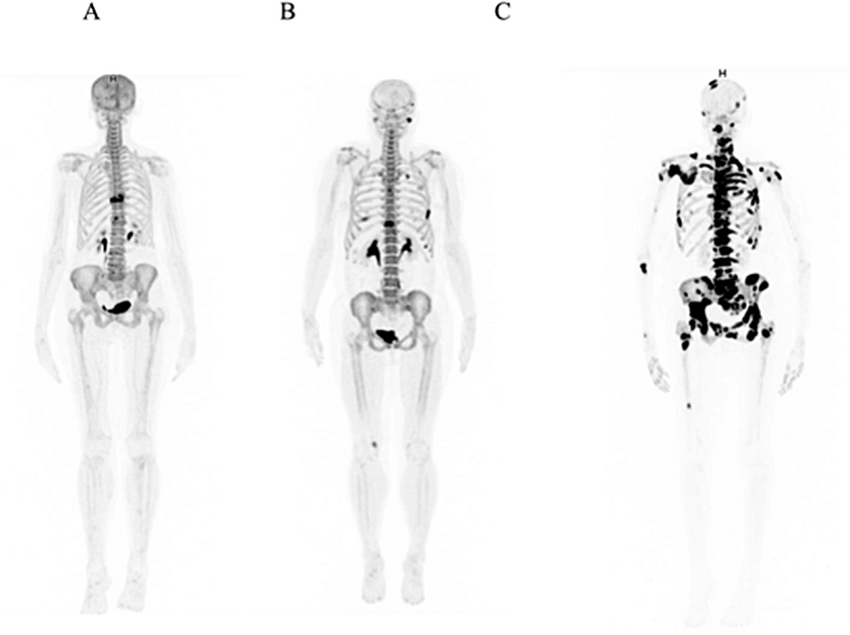
- The primary radio-imaging agent used in PET scanning is 18F-FDG.
- Examples include research on the heart, where the myocardium is evaluated by using the technique to distinguish between living and dead tissue. It can be used in neurology to diagnose brain tumors, dementia, and seizure disorders. Typically, 18F-FDG is utilized to evaluate a cancer patient’s tumor’s extent. Increased cell growth, which needs energy and thus more glucose, is a characteristic of cancerous tissue. This causes malignant tumors to accumulate 18F-FDG, which can be used to gauge the extent of metastasis. Both the initial evaluation of the cancer stage and any surgical operation require this knowledge.
- Unfortunately, because 18F-FDG has a non-specific uptake, its usage is limited. Consequently, misdiagnosis may result from a buildup of 18F-FDG caused by other diseases. These ailments include inflammation and wound healing, both of which exhibit elevated glucose metabolism.
- As a result, several different 18F-labelled substances, mostly those with a more specialized biological route, are being closely examined as potential substitutes for PET scanning agents.
- Among them is 18F-choline. Due to choline’s incorporation into the cell membrane, cells that divide quickly require more of this material. While research was done on a variety of tumors, the majority of the studies focused on prostate cancer. When compared to 18F-FDG, 18F-choline exhibited reduced bladder activity and a longer renal elimination period. Furthermore, fast cell division is a physiologic phenomenon unrelated to cancer that can result in a false diagnosis.
67Gallium: PET
- Gallium is made up of two stable isotopes (69Ga and 71Ga) and two commercially available radioisotopes (67Ga and 68Ga).
- The half-life of 67Ga is 3.3 days, whereas that of 68Ga is considerably shorter at 68 minutes.
- Gamma cameras can detect the γ-rays released by 67Ga during its electron capture decay.
- PET uses 68Ga, an isotope that emits positrons. Generators are used to obtain fresh 68Ga for clinical applications due to its short half-life. The parent molecule, 68Ge, is used in the generator. It has a half-life of 271 days and breaks down into its “daughter,” 68Ga, through electron capture.
- When injected into animals with tumors, it has been seen that radioactive gallium-67 citrate accumulates in malignant cells. 67Ga scans were created as a result, and throughout the past 20 years, they have primarily been used to find cancer cells that remain in patients with Hodgkin’s and non-Hodgkin’s lymphomas following chemotherapy or radiation treatment. The quantity of 67Ga found in lymphoma cells is closely correlated with both the rate of their proliferation and their metabolic activity. Consequently, a positive 67Ga scan (usually performed following chemotherapy) suggests that cancerous cells are still alive and that additional therapy is required.
- Transferrin is mostly responsible for moving Ga3+. According to in vitro research, adding transferrin to the media dramatically enhanced the cancer cells’ absorption of radioactive gallium.
201Thallium
- Hallium is an element with the atomic number 81 and the chemical symbol Tl. It is a member of the boron group.
- The soft grey metal thallium is not present in nature as a free metal.
- The two most prevalent oxidation states for thallium ions are +1, which is the much more dominant oxidation state, and +3, which is similar to the oxidation states of other group members. In biological systems, thallium ions with an oxidation state of +1 behave chemically like alkali metals and are treated similarly to potassium (K+) ions.
- Many of the chemicals including thallium are hazardous. In instance, because of its behavior like that of K+, the Tl+ cation exhibits strong aqueous solubility and can enter the body through potassium-based absorption pathways.
- Unfortunately, the chemistry of the two ions differs, which has an impact on things like how well they attach to molecules that contain sulfur and causes thallium ions to be hazardous.
- Rat poison was once made using chemicals based on thallium, but due to their general poisonous nature, this practice has been abandoned.
- Thorium poisoning can cause hair loss, neurological damage, and in extreme cases, abrupt death at high enough concentrations.
- The primary element employed in nuclear imaging for cardiology was the radioactive isotope thallium-201 (201Tl). This substance played a key role in the thallium nuclear cardiac stress test, wherein a radiotracer like 201TlCl (thallous chloride-201) was administered to a patient during physical activity. Following a brief waiting period to ensure optimal distribution of the radioactive material, the heart was imaged using a gamma camera to assess blood flow within the cardiac muscle. In contemporary practice, 99mTc imaging has largely supplanted the use of the 201Tl isotope.
- The isotope 201Tl, with a half-life of 73 hours, can be produced through a portable thallium-201 generator. This generator utilizes 201Pb (lead-201) as the ‘parent’ substance, undergoing electron capture decay to produce the ‘daughter’ isotope 201Tl. The decay of 201Tl by electron capture yields favorable imaging characteristics
Video on Radiopharmaceuticals
References
- Akul Munjal; Nishant Gupta; Radiopharmaceuticals.
- Filipe Boccato Payolla, Antonio Carlos Massabni, and Chris Orvig; Radiopharmaceuticals for diagnosis in nuclear medicine: a short review.
- George Crișan, Nastasia Sanda Moldovean-Cioroianu, Diana-Gabriela Timaru, Gabriel Andrieș, Călin Căinap,3 and Vasile Chiș; Radiopharmaceuticals for PET and SPECT Imaging: A Literature Review over the Last Decade.
- https://openmedscience.com/radiopharmaceutical/