Rutherfordium is a synthetic transition metal with an atomic number of 104 and is represented by the symbol ‘Rf’ in the periodic table. It is silvery in appearance and belongs to the d-block of period 7 of the periodic table. Rutherfordium was the first transactinide (super-heavy) element identified.
It’s possible that Rutherfordium was first created in 1964 when a group of Russian scientists under Georgy Flerov’s direction bombarded a plutonium target with neon ions. Researchers at the University of California, Berkeley, led by Albert Ghiorso, successfully synthesized rutherfordium in 1969 by bombarding a target of californium with carbon-12 and carbon-13 ions.
Interesting Science Videos
Discovery and History of Rutherfordium
- Georgy Flerov and his colleagues in Dubna, Russia identified the element 104 independently, as did Albert Ghiorso and his colleagues in Berkeley, California, USA.
- Flerov’s team blasted plutonium using neon during 1964, producing the element’s isotope 259 at Russia’s Joint Institute for Nuclear Research (JINR). They confirmed their discovery in 1966.
- In honor of Igor Kurchatov (1903–1960), the head of the Soviet atomic bomb project, the JINR group recommended calling the project Kurchatovium (symbol Ku).
- Berkeley researchers successfully created three distinct isotopes of rutherfordium in 1969 by bombarding curium-248 with oxygen-16 to produce isotope 260, californium-249 with carbon-12 to produce isotope 257, and californium-249 with carbon-13 to produce isotope 258.
- The Berkeley team proposed the element name rutherfordium (sign Rf) in a discovery claim.
- So the element 104 ended up with two different names for several decade.
- In 1997, the IUPAC working group judged that both the JINR and Berkeley groups had some basis for their discovery claims and designated element 104 as rutherfordium.
Occurrence of Rutherfordium
- Rutherfordium is not found naturally in the earth’s crust.
- It is a synthetic radioactive metal generated through the bombardment and decay of heavy isotopes.
- Rf can be produced by bombarding plutonium-242 with accelerated neon ions or bombarding californium-249 with accelerated carbon ions.
- Rutherfordium contains 15 isotopes having known half-lives ranging in mass from 253 to 268. None are stable.
Elemental Properties of Rutherfordium
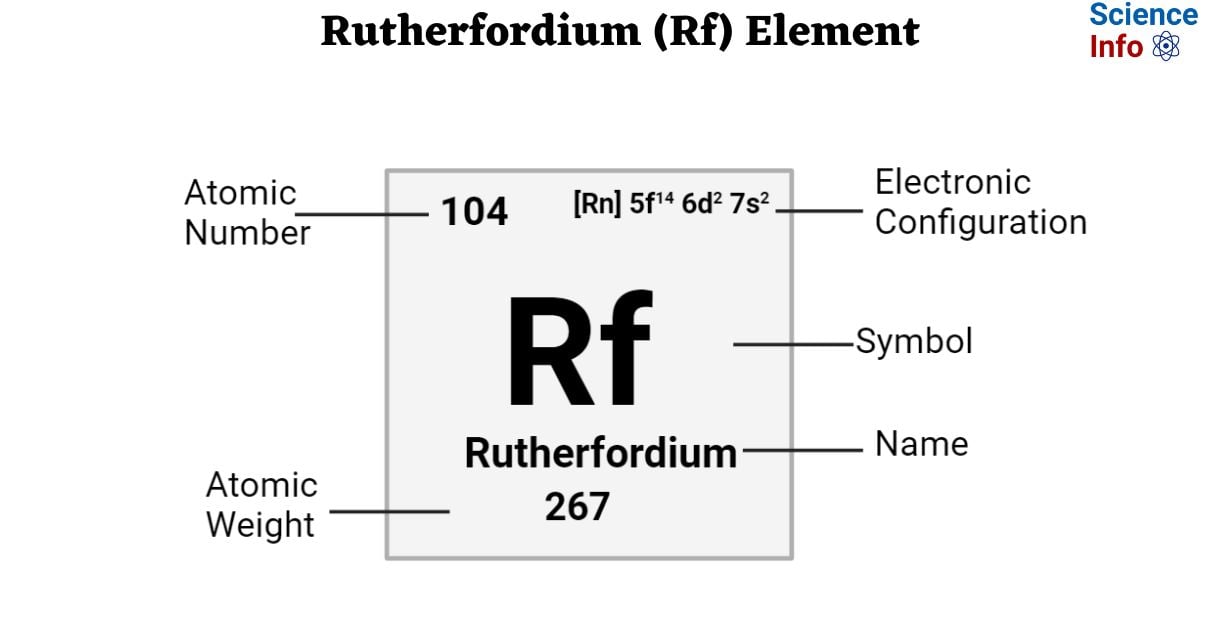
| Electronic Configuration | [Rn] 5f14 6d2 7s2 |
| Atomic Number | 104 |
| Atomic Weight | 267 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Trans-actinides, 7, d-block |
| Density | – |
| Ionic radius | – |
| Van der Waals radius | – |
| Electron shells | 2, 8, 18, 32, 32, 10, 2 |
| Electrons | 104 |
| Protons | 104 |
| Neutrons | 163 |
Isotopic Information of Rutherfordium
- Rutherfordium contains no stable isotopes, and its isotopes do not occur naturally.
- It’s isotopes, which are radioactive, have been created in a laboratory by fusing two elements and decaying heavier elements.
- Lighter isotopes, such as 253-Rf and 254-Rf, have shorter half-lives of approximately 50µs. 256-Rf, 258-Rf, and 260-Rf are steady at 10 ms.
- While 255-Rf, 257-Rf, 259-Rf, and 262-Rf have half-lives ranging from 1 to 5 seconds. The half-life of 261Rf is one minute, 1.5 minutes for 265-Rf, and ten minutes for 263-Rf.
- The heaviest isotope 267-Rf has a half-life of approximately 1.3 hours.
Physical Properties of Rutherfordium
- The physical characteristics of rutherfordium, as a synthetic element, are largely unknown.
- It is found in the 7th period, the 4th Group, and the d-block of the periodic table.
- It is referred to as the initial transactinide atom. It is extremely radioactive, in a solid state, and shares characteristics with hafnium.
- It has a 150 pm atomic radius.
- According to current theories, it is a silvery, thick metal with a high melting and boiling points.
- Rutherfordium is extremely radioactive, and its isotopes have short half-lives, making it challenging to research.
- Boiling Point of the element 104 is unknown.
- Melting Point of the Rf is unknown.
Chemical Properties of Rutherfordium
- Rutherfordium is a synthetic, extremely reactive element. It is the first transactinide element (very heavy).
- It has chemical characteristics similar to elements in the 4th group of the periodic table, particularly Hafnium (Hf).
- Rutherfordium’s most stable oxidation state is +4, however it is thought to be capable of producing less stable compounds at +3.
- It has a hexagonal closed-packed crystal structure.
- Element 104 interacts with halogens to generate tetrahalides RfX4, which are then hydrolyzed in water to form oxyhalides RfOX2. Tetrahalides are volatile solids that exist in vapor form as monomeric tetrahalide molecules.
- Rutherfordium has 12 known isotopes, each with their own half-life.
- The most stable of the 12 isotopes, 265-Rf, has a half-life of around 13 hours and can be degraded via fission.
Uses of Rutherfordium
- Rutherfordium has no practical usage other than scientific research because of its extreme radioactivity.
- Its principal application is the study of nuclear physics and the properties of heavy elements.
- Rutherfordium has been used in studies to investigate the formation of super-heavy elements, and its properties have been utilized to better understand the behavior of other elements in the same group.
- Rutherfordium is a synthetic chemical element with intriguing features that make it a valuable tool for studying nuclear physics and heavy element qualities.
- It has no practical applications outside of scientific research due to its extreme radioactivity, but its study is critical for furthering our understanding of the universe and matter’s behavior at the atomic and subatomic levels.
Health Effects of Rutherfordium
- Because it is so unstable, any amount produced could break down into other elements so rapidly that there is no reason to investigate its effects on human health.
Environmental Effects of Rutherfordium
- Rutherfordium’s environmental ramifications are unnecessary due to its relatively short half-life (about 10 minutes).
Video Reference
References
- https://www.theguardian.com/science/grrlscientist/2013/sep/20/chemistry-chemistry
- https://eduinput.com/rutherfordium/
- https://collegedunia.com/exams/rutherfordium-chemistry-articleid-2331
- https://www.vedantu.com/chemistry/rutherfordium
- https://www.chemistrylearner.com/rutherfordium.html
- https://www.lenntech.com/periodic/elements/rf.htm
- https://www.chemicool.com/elements/rutherfordium.html

