The Stephen reaction, also referred to as the Stephen reduction or Stephen aldehyde synthesis, is a chemical process that transforms a nitrile into its corresponding aldehyde in the presence of hydrochloric acid. Essentially, it is an organic redox reaction pioneered by the English chemist Henry Stephen. In this significant name reaction of organic chemistry, nitriles undergo reduction to imines using tin chloride and hydrochloric acid, followed by hydrolysis to yield aldehydes. The by-products of the Stephen reaction include ammonia and ammonium chloride.
Interesting Science Videos
What is Stephen Reaction?
The Stephen reaction, also known as Stephen Aldehyde Synthesis or Stephen Reduction, is a crucial organic redox process named after English chemist Henry Stephen. This reaction involves the conversion of nitriles to aldehydes in the presence of hydrochloric acid, using tin chloride. The process includes the reduction of nitriles to imines, quenching of the resulting iminium salt, and hydrolysis to yield aldehydes. Ammonium chloride and ammonia are produced as side products.
First reported in 1925, the Stephen reaction is a significant name reaction in organic chemistry, and its success is now known to depend on factors such as the formation of the crystalline “aldimine stannichloride.” While the reaction can yield almost quantitative yields, it is generally more effective for aromatic nitriles than aliphatic ones, and certain aldehydes may not be synthesized efficiently using this method.
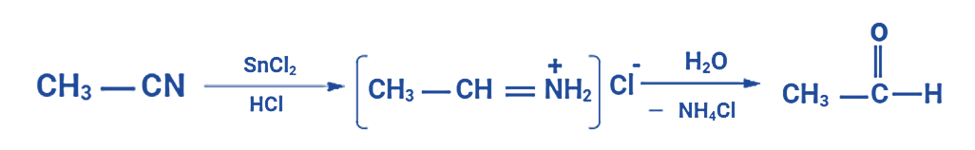
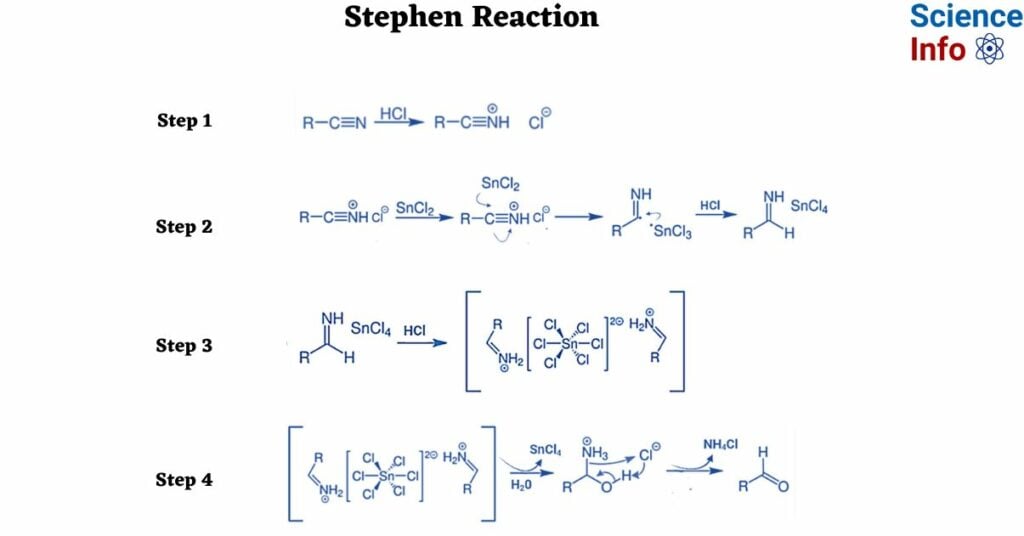
Stephen Reaction Mechanism
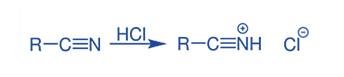
Step 1: In the initial step, introduce gaseous hydrogen chloride to the provided nitrile, initiating a reaction that results in the formation of the corresponding salt as illustrated below.
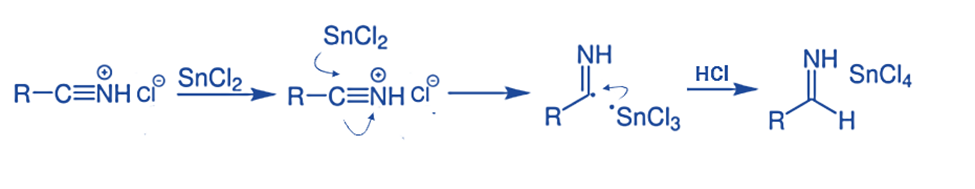
Step 2: The subsequent step entails reducing the aforementioned salt. Tin chloride serves as a reducing agent, providing a single electron during the process. This then reacts with HCl to produce another salt.
Step 3: This involves the rapid precipitation of the salt obtained in the second step, leading to the formation of aldimine tin chloride, as demonstrated below.
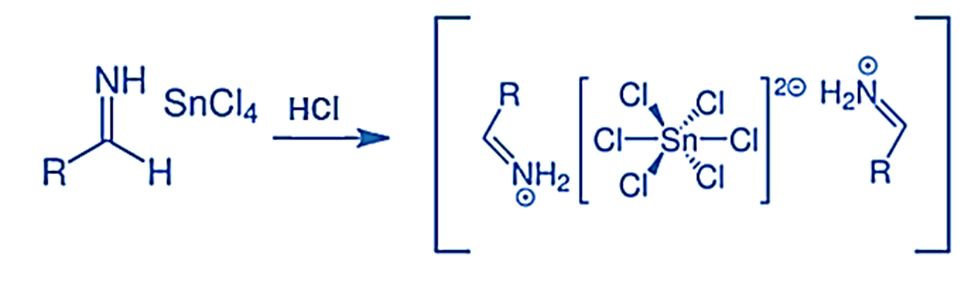
Step 4: In the fourth step, the hydrolysis of aldimine tin chloride results in the production of an amide. The subsequent formation of the necessary aldehyde occurs from this amide, as indicated below:
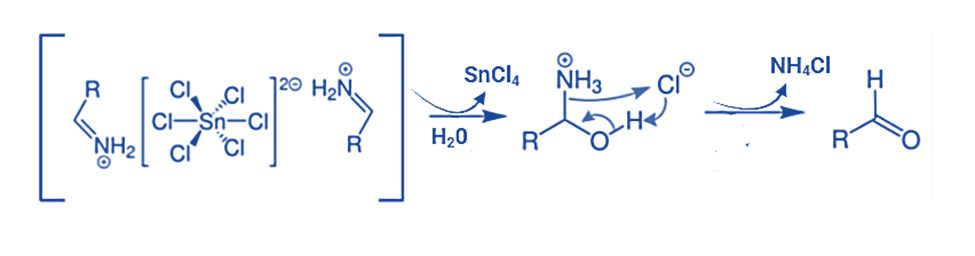
In Stephen’s reaction mechanism, the ultimate result is the production of an aldehyde. It is crucial to note that the utilization of aliphatic and aromatic nitriles proves to be more effective. Additionally, enhancing electron density by incorporating substitutes enhances the tin chloride salt formation. This effect can be further promoted by the introduction of electron-withdrawing substances, such as the formation of amide chloride.
Stephen Reaction Example
A notable illustration of the Stephen aldehyde synthesis involves generating acetaldehyde from methyl cyanide. In this process, the nitrile undergoes reduction through the action of hydrochloric acid and tin(II) chloride, as previously described, leading to the formation of an imine intermediate. Subsequently, this imine intermediate experiences hydrolysis, ultimately yielding the desired aldehyde—in this instance, acetaldehyde.
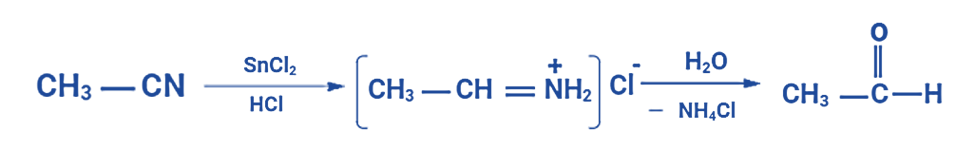
References
- https://byjus.com/chemistry/stephen-reaction-mechanism/#
- https://www.pw.live/concepts-stephens-reduction
- https://collegedunia.com/exams/stephen-reaction-mechanism-chemistry-articleid-5131
- https://protonstalk.com/aldehydes-ketones-carboxylic-acids/stephen-reaction/
- https://testbook.com/chemistry/stephen-reaction-mechanism
