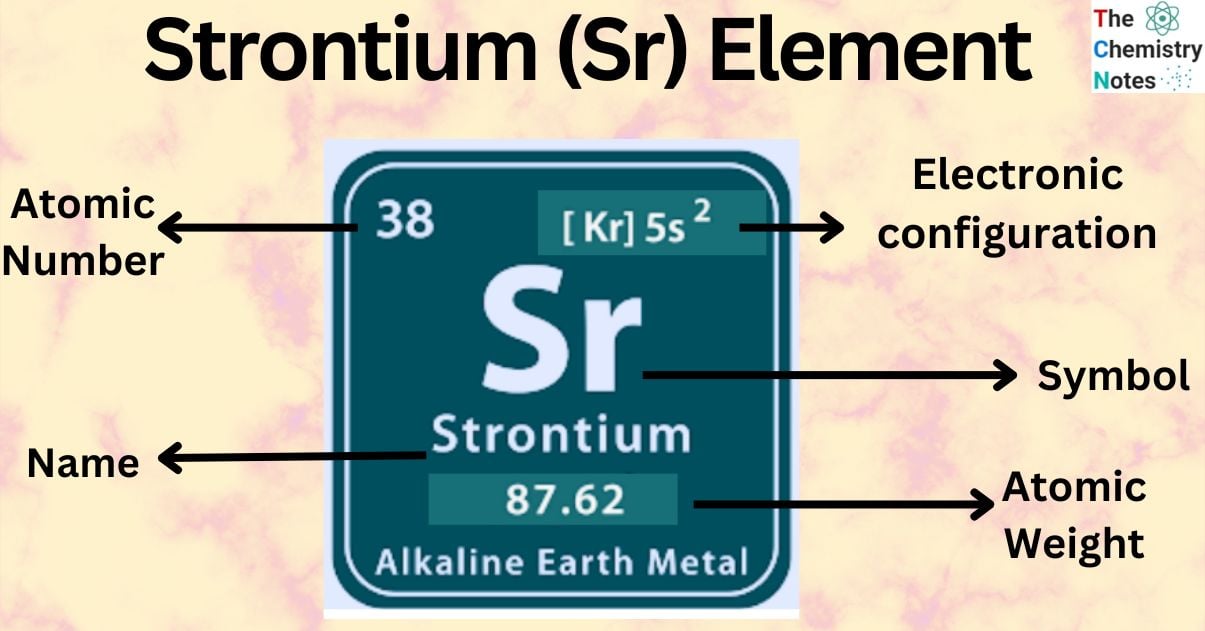
Strontium is a chemical element with the atomic number 38 and is represented by the symbol ‘Sr’ in the periodic table. It belongs to the s-block of a group 2 of the periodic table along with Beryllium, Magnesium, Calcium, and Barium and is one of the alkaline earth metal. It is a soft silver-white-yellowish metallic element that is highly chemically reactive.
Strontium is a common element found in nature, ranking as the 15th most abundant element in the Earth’s crust. It shares physical and chemical features with its two vertical periodic table neighbors, calcium and barium. It is typically obtained from the minerals celestine and strontianite, which occur naturally.
Interesting Science Videos
History of Strontium
- Adair Crawford (an Irish chemist) was the first to discover strontium in the year 1790 during the research of witherite (BaCO3).
- Crawford mixed the mineral and hydrochloric acid (HCl), but the final result was not as expected.
- Because of the failure of the experiment, he assumed that his sample (witherite) was contaminated with an unknown mineral, which he eventually termed strontianite (SrCO3).
- Sir Humphry Davy, an English chemist, was the first to isolate strontium in 1808. He used the electrolysis of a mixture of chloride (SrCl2) and mercuric oxide (HgO).
- The element got its name from the small Scottish village “Strontian, which was the place where it was discovered in the ore of the lead mines.
Occurrence of Strontium
- Strontium is commonly found in nature; it is the fifteenth most abundant element on the Earth’s crust. It is estimated to be approximately 360 ppm in the Earth’s crust.
- It is most typically found in the sulfate minerals celestine (SrSO4) and strontianite (SrCO3). Celestine is far more prevalent in deposits large enough for mining.
- Strontium readily reacts with air and water, so it only exists in the form of a mineral in nature.
- The leading producers of strontium are China, Mexico, Spain, Turkey, and the United Kingdom, among others.
Isotopes of Strontium
Strontium has four naturally occurring stable isotopes: 84Sr, 86Sr, 87Sr,and 88Sr
Naturally occurring isotopes
| Isotopes | Natural abundance (atom %) |
|---|---|
| 84Sr | 0.56 (1) |
| 86Sr | 9.86 (1) |
| 87Sr | 7.00 (1) |
| 88Sr | 82.58 (1) |
Elemental Properties of Strontium
| Electronic Configuration | [Kr] 5s2 |
| Atomic Number | 38 |
| Atomic Weight | 87.62 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 2, 5, s-block |
| Density | 2.64 g cm -3 at 20 °C |
| Ionic radius | 0.113 nm (+2) |
| Van der Waals radius | 0.215 nm |
| Electron shells | 2,8,18,8,2 |
| Electrons | 38 |
| Protons | 38 |
| Neutrons in most abundant isotope | 50 |
Physical Properties of Strontium
- Strontium has an atomic number of 38 and is a soft silvery-white shiny metal. However, when exposed to air, it reacts with oxygen to generate a thin strontium oxide (SrO) coating. The coating gives the metal a yellowish color.
- Strontium has a melting point of 777°C (1431°F) and a boiling point of 1377°C (2511°F).
- It is in a solid state at room temperature.
- The density of Strontium is 2.6 grams per cubic centimeter.
- Strontium is malleable, which means it can be drawn into a thin wire without breaking it, but it does have very little use in its elemental form.
- Strontium is a semi-conductor of electricity. Its electrical conductivity is comparable to that of rubidium and chromium.
- Strontium is odorless.
| Color/physical appearance | Silvery–white |
| Melting point/freezing point | 777°C, 1431°F, 1050 K |
| Boiling point | 1377°C, 2511°F, 1650 K |
| Density | 2.64 g cm-3 at 20°C |
Chemical Properties of Strontium
- Strontium is a divalent metal.
- It is a highly reactive metal, so when it is exposed to air, an oxide layer forms on the surface.
- At normal room temperature, finely ground strontium spontaneously ignites, emitting a bright red flame.
- Because of strontium’s strong reactivity, it must be kept in mineral oil or kerosene.
- Strontium can react with hydrogen and nitrogen when it is heated.
Chemical Reaction Of Strontium
- Reaction of Strontium with Water
Strontium slowly interacts with water, producing strontium hydroxide, Sr(OH)2, and hydrogen gas, H2. The strontium metal sinks in water, and after a short time, bubbles of hydrogen appear on the metal’s surface. The reaction is faster than that of calcium but slower when compared to barium.
Sr (s) + 2H2O (g) → Sr(OH)2 (aq) + H2 (g)
- Reaction of Zinc with Air
Strontium is a white metal that features a silvery luster. When it is ignited in the air, a mixture of white strontium oxide, SrO, and strontium nitride, Sr3N2, is produced. The surface of the metal is then covered with a thin layer of oxide, which protects it from air attack to a lesser degree than the similar coating in magnesium.
2Sr (s) + O2 (g) → 2SrO (s)
3Sr (s) + N2(g) → Sr3N2 (s)
- Reaction of Strontium with the Halogens
Strontium is highly reactive to halogens.
Strontium reacts with chlorine, Cl2, and burns to form the dihalide strontium (II) chloride, SrCl2.
Sr (s) + Cl2 (g) → SrCl2 (s)
Strontium reacts with bromine, Br2, and burns to form the dihalide strontium(II) bromide, SrBr2, and this reaction takes place at about 400°C.
Sr (s) + Br2 (g) → SrBr2 (s)
Strontium reacts with iodine, I2, and burns to form the dihalide strontium(II) iodide, SrI2, and this reaction takes place at dull red heat.
Sr (s) + I2 (g) → SrI2 (s)
- Reaction of Strontium with Acids
Strontium metal quickly dissolves in dilute or strong hydrochloric acid, forming solutions containing the aqueous Sr(II) ion and hydrogen gas, H2.
Sr (s) + 2HCl (aq) → Sr2+ (aq) + 2Cl– (aq) + H2 (g)
Uses Of Strontium
The use of strontium in its elemental form is rare. But it can be used in different industries that include:
Used In Sugar Industries: The processing of raw sugar was one of the earliest applications for strontium, and it is still used today. To extract sugar from sugar beets and remove molasses from raw sugar, strontium hydroxide is employed. This strontium compound was formerly manufactured from the strontium minerals strontianite and celestite.
Used In Glass For Cathode Ray Tubes: Previously, strontium was primarily utilized in color television cathode-ray tubes (CRTs). They are still manufactured, but they are becoming less common. Strontium oxide is mixed with glass and absorbs the X-rays emitted by the CRT. This glass is mostly used for the display surface of the tube instead of the entire tube.
Used In Pyrotechnics: One of the most common applications for strontium is in pyrotechnics. Because of its characteristics, it can be used to produce specific brilliant red colors in fireworks, emergency flares, and tracer bullets. Strontium emits an exceptionally brilliant, red-colored light.
Use In Medical Science: Strontium can also be used in medication. While research is ongoing, it is believed that specific strontium compounds can help prevent, treat, and even reverse bone loss. Radioactive strontium isotopes are also used to treat various cancers.
Used In Vacuum Space: Strontium is also used as a getter. Because it is a highly reactive metal, it can be used to get rid of unwanted gases from the vacuum space.
Used In Refining: Strontium metal is used in the refining of zinc to remove trace levels of lead impurities.
Used In Alloys: Although the metal is malleable, ductile, and an excellent conductor of electricity, elemental strontium has limited applications. One of them is used in cast-block engines and wheels as an alloying agent for aluminum or magnesium; the strontium increases the metal’s machinability and creep resistance.
Used In Paints And Plastics: Strontium aluminate (SrAl2O4) is used in current glow-in-the-dark paints and polymers. They absorb light during the day and gently release it thereafter for hours. It is also used in toys.
Strontium is also utilized in the pigmentation of paints and the glaze of ceramics. It is also occasionally used in toothpaste.
Health Effects Of Strontium
- Low levels of (radioactive) strontium are sometimes ingested through breathing in air or dust, eating food, drinking water, or coming into touch with strontium-containing soil. It is more likely to enter the human body through food or drink.
- Strontium chromate (SrCrO4) is the only strontium compound that is toxic to humans, even in trace concentrations. This is mostly related to the presence of toxic chromium.
- Strontium is regarded as safe in normal food levels and as a component in toothpaste. In general, there isn’t enough evidence to indicate whether any type of Sr is safe for pregnant or nursing mothers, therefore it’s recommended to avoid using strontium supplements in these situations.
- Strontium’s positive effect on diseases such as osteoporosis, bone cancer, prostate cancer, tooth sensitivity, and tooth decay has been recognized by scientific and medical societies.
- The safest possible way to boost the strontium levels in your body is by obtaining it from your diet. Whole grains, beans, carrots, potatoes, spinach, celery, and seafood are some of the best food supplements for strontium.
Environmental Effects of Strontium
- Strontium is naturally occurring element throughout the Earth’s crust.
- High concentrations of Strontium in water and soil can be caused by human activities or industrial waste.
- Due to the high concentration of strontium in water and the soil, it can end up in the fish, livestock, and vegetables that we consume daily.
- Nuclear blasts and nuclear leakage may cause the radioactive strontium isotope to be released into nature, causing various problems for the environment and humans.
- Strontium can affect aquatic life when it is highly concentrated.
Watch out for the video for interesting information related to Strontium. Warning! Some experiments are dangerous, do not try it by yourself!
References
- MacMillan, J. Paul; Park, Jai Won; Gerstenberg, Rolf; Wagner, Heinz; Köhler, Karl and Wallbrecht, Peter (2002) “Strontium and Strontium Compounds” in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim. doi:10.1002/14356007.a25_321
- Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- https://www.lenntech.com/periodic/elements/sr.htm#:~:text=Strontium%20is%20a%20soft%2C%20silver,contact%20with%20air%20and%20water.
- https://www.rsc.org/periodic-table/element/38/strontium
- https://www.britannica.com/science/strontium
- https://pubchem.ncbi.nlm.nih.gov/element/Strontium
- https://www.chemicool.com/elements/strontium.html
- Mary Elvira Weeks, Discovery of the Elements., Journal of Chemical Education (June 1932) p1046.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8

