Tennessine is a synthetic transition metal with an atomic number of 117 and is represented by the symbol ‘Ts’ in the periodic table. It is silvery in appearance and belongs to the p-block of period 7 of the periodic table. It is the penultimate element in the periodic table. Only tiny quantities of Tennessine have been successfully synthesized, hence there isn’t much known about it based on experimental data, but some qualities can be predicted using periodic table trends.
Tennessine is an extremely radioactive element that does not occur naturally, and is produced inside a laboratory setting and decays within milliseconds after being synthesized. This element, formerly known as Ununseptium (Uus) or eka-astatine, was created in 2010 by a group of American scientists working at the Lawrence Livermore National University (USA), Russian Scientist working at Joint Institute for Nuclear Research (JINR) in Dubna, Russia and Oak Ridge National Laboratory (ORNL) Tennessee. Tennessine is named after the US state of Tennessee, where the Oak Ridge National Laboratory (ORNL) is based. In 2016, the element was officially added to the periodic table.
Interesting Science Videos
History and Discovery of Tennessine
- Tennessine was discovered through a collaborative effort involving the Joint Institute of Nuclear Research (JINR), Lawrence Livermore National Laboratory (LLNL), and Oak Ridge National Laboratory.
- The Oak Ridge National Laboratory (ORNL) was responsible of synthesizing its target material, berkelium, which took around two hundred and fifty days to yield 22 milligrams.
- The berkelium was subsequently shipped to the Research Institute of Atomic Reactors (RIAR) and set as a 300 nm thin layer on a titanium film.
- Afterwards it, it was taken to JINR and placed in the particle accelerator. The target was bombarded with calcium-48, and the experiment’s results were forwarded to Lawrence Livermore National Laboratory (LLNL), for further evaluation.
- The first bombardment lasted seventy days. The berkelium was attacked with almost 7 trillion calcium-48 ions per second, which accelerated it to around 10% of the speed of light.
- A Russian-American collaboration revealed their discovery of element 117 in 2010.
- The same team corroborated their findings in 2012, and a German-American team successfully replicated the experiment in 2014.
- The element’s discovery involved two different nations and multiple research centers, therefore naming could have been difficult.
- However, several new elements were validated, making it easier to settle on a name.
- The Russian-American team proposed the new name Tennessine for element 117 to honor the work of Oak Ridge National Laboratory from Tennessee. The symbol is Ts, which is the abbreviation for Tennessee’s state name.
Occurrence of Tennessine
- Tennessine can be synthesized artificially. It’s a synthetic element that is extremely unstable. Its half-life is only a few seconds.
- Ts is a synthetic radioactive metal formed by nuclear bombardment and has only been manufactured in trace amounts. And it is only found in specialized laboratories due to its rapid decay.
- Tennessine can be synthesized by blasting 249Bk with 48Ca in a heavy ion accelerator.
- According to an article published in Physical Review Letters on April 5, 2010 (“Synthesis of a new chemical element with atomic number Z=117”), the isotopes 293Ts (five atoms) and 294Ts (one atom) were identified in fusion processes involving 48Ca and 249Bk.
4820Ca + 24997Bk → 297117Ts → 293117Ts + 4 n
4820Ca + 24997Bk → 297117Ts → 294117Ts + 3 n- Initial research has discovered two isotopes of tennessine, with mass numbers 293Ts and 294Ts.
Elemental Properties of Tennessine
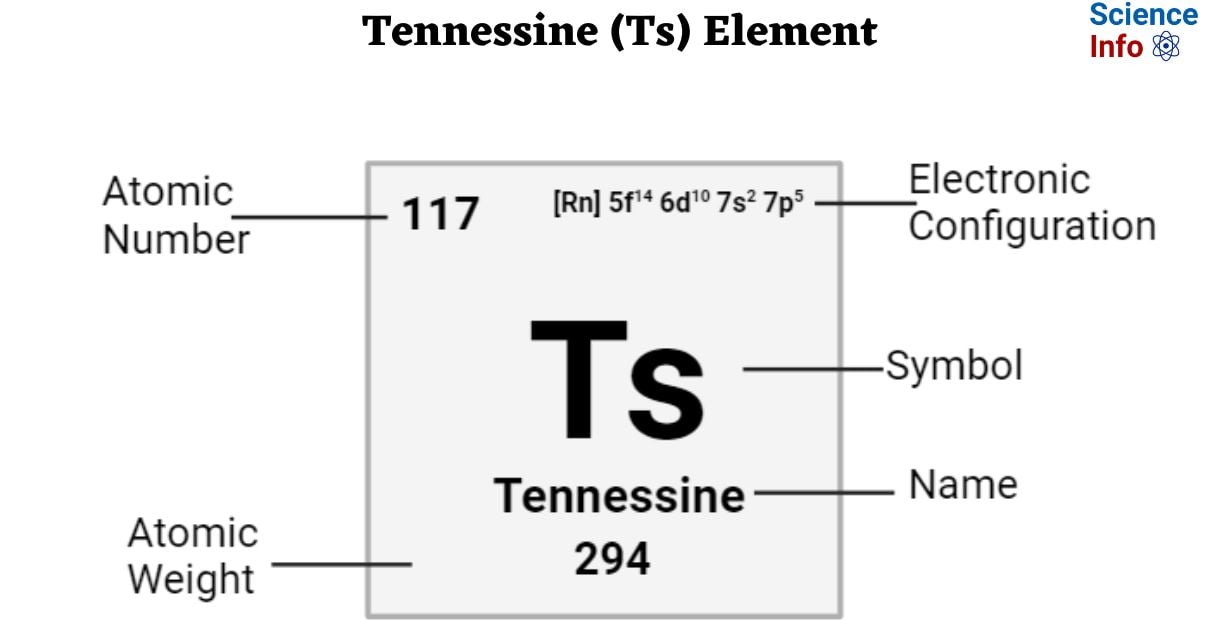
| Electronic Configuration | [Rn] 5f14 6d10 7s2 7p5 |
| Atomic Number | 117 |
| Atomic Weight | 294 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Transactinide, 7, p-block |
| Density | 7.1 g/cm3 -7.3 g/cm3 (estimated) |
| Ionic radius | – |
| Van der Waals radius | – |
| Electron shells | 2, 8, 18, 32, 32, 18, 7 (estimated) |
| Electrons | 117 |
| Protons | 117 |
| Neutrons | 177 |
Isotopic Information of Tennessine
- Tennessine does not have any naturally occurring stable isotopes, but they can be created in a laboratory setting.
- All of the Ts isotope are unstable and radioactive.
- Also isotopes of Ts decay via alpha decay or spontaneous fission, but none of them undergo beta decay.
- Tennessine has two known isotopes: 293Ts and 294Ts.
- Lv-293 has a half-life of about 0.6 seconds and is currently the most stable isotope of livermorium known to scientists.
- Tennessin-293 has a half-life of 14 milliseconds, whereas tennessin-294 has a half-life of 78 milliseconds.
- Tennessine-293 decays by emitting alpha particles into element moscovium, which is element 115.
Physical Properties of Tennessine
- Tennessine’s instability makes it difficult to conduct an objective examination of its physical properties.
- Given its swift disintegration, only a few properties of tennessine have been studied to date.
- It is a synthetic, super-heavy transactinide element.
- It is found in the 7th period, the 17th Group (halogens), and the p-block of the periodic table.
- Though it hasn’t been proven to function as a heavier homologue of the chalcogens , it is categorized as the heaviest chalcogens in group 16.
- Considering its position on the periodic table, element 117 can be expected to be a halogen, such as chlorine or bromine.
- Tennessine should resemble astatine, the element located above it on the periodic table, more than any other.
- While element 117’s chemical behavior may differ from that of halogens, physical qualities such as melting and boiling points are expected to follow halogen trends.
- The atomic mass of Tennessine is anticipated to be 294. (The atomic mass of man-made trans-uranium elements is calculated using the periodic table’s longest-lived isotope. These atomic weights should be considered tentative because a new isotope with a longer half-life may be created in the future).
- Tennessine, is anticipated to be a solid at ambient temperature like astatine.
- A density of around 7.1 g/cm3 is anticipated for tennessine.
- The isotope of Tennessine with the longest half-life is 0.78 seconds.
Chemical Properties of Tennessine
- Tennessine is a highly radioactive element. Its chemical properties have yet to be completely investigated. Isotopes have short half-lives, and the compounds they contain are highly volatile, making conclusive chemical analysis challenging.
- There have been no experimental measurements of tennessine compounds, and all known predictions are theoretical.
- It is the heaviest element in the 17th group of the periodic table.
- Tennessine, unlike the lighter group 17 elements, may not display the chemical behavior associated with halogens. Fluorine(F), Chlorine, Bromine (Br), and Iodine (I), for example, commonly absorb an electron to gain a noble gas’s more stable electronic configuration, which creates eight electrons (octets) in their valence shells rather than seven.
- Tennessine is expected to act similarly to other halogens in the absence of experimental data. It might mostly resemble polonium.
- It is anticipated that Tennessine may have different properties than the lighter members of its group due to the orbit-spin interaction and relativistic effect.
- It is projected to be a volatile metal that has low oxidation states in the compounds -1, +1, +3, and +5.
- Tennessine compounds are projected to exhibit +5 and +3 as the least stable oxidation states.
- Tennessine molecules are projected to have the least stable oxidation state -1.
- It is thought to have different properties than halogens due to relativistic effects. However, its ionization energy, boiling temperature, and melting point will be consistent with that of halogens.
Chemical Reaction of Tennessine
The reactions of tennessine are not conclusive.
- Reaction of Tennessine with Air
Merely a handful of atom of tennessine are known to have been created, hence its reactivity with air is undetermined. One would expect its behavior to be comparable to that of astatine (directly above tennessine in the periodic table) and iodine (two places above).
- Reaction of Tennessine with Water
Just a couple of atom of tennessine were ever created, so its interaction with water is unknown. One would expect its behavior to be comparable to that of astatine (directly above tennessine in the periodic table) and iodine (two places above).
- Reaction of Tennessine with Halogens
Perhaps just a few atom of tennessine have previously been created, therefore its reactivity with halogens is unknown. One would anticipate its behavior to be comparable to that of astatine (directly above tennessine in the periodic table) and iodine (two places above).
- Reaction of Tennessine with Acids
Apparently just a few atoms of tennessine have been isolated and created, thereby its reactivity with acids is untested. One would expect its behavior to be comparable to that of astatine (directly above tennessine in the periodic table) and iodine (two places above).
Synthesis of Tennessine
- All elements with atomic numbers more than 100 can only be created through reactions in a particle accelerator, such as a cyclotron; they do not develop in a nuclear reactor.
- When Berkelium-249 is bombarded with calcium-48, it results in tennessine-293 and tennessine-294.
Uses of Tennessine
- Given that few atoms of this metal have been synthesized thus far, there are currently no specific or specialized applications for tennessine other than scientific research.
- A constant experimental study aimed at achieving an obvious conclusion requires a large number of atoms. Perhaps a few tennessine atoms have been produced thus far.
Health Effects of Tennessine
- Tennessine is a highly unstable chemical; when created, it swiftly decomposes into other elements, therefore it has no impact on human health. However, being a radioactive element it must be toxic.
Environmental Effects of Tennessine
- Tennessine’s environmental effects are negligible due to its short half-life (just a few seconds).
Video Reference
References
- https://www.thoughtco.com/element-117-facts-ununseptium-or-uus-3880071
- https://chemicalengineeringworld.com/tennessine-element-properties-and-information/
- https://phys.org/news/2016-11-tennessine-element.html
- https://www.webelements.com/tennessine/chemistry.html
- https://periodic-table.com/tennessine/

