The X-ray fluorescence (XRF) spectrometer is an analytical instrument that employs X-ray technology to perform routine and minimally invasive chemical analyses of various geological materials such as rocks, minerals, sediments, and fluids. The operational principles of this system are based on wavelength-dispersive spectroscopy, which shares similarities with the electron microprobe (EPMA) technique. Nevertheless, X-ray fluorescence (XRF) instruments are generally not capable of conducting analyses at the small spot sizes commonly used in electron probe microanalysis (EPMA) work, which typically range from 2 to 5 microns. As a result, XRF is commonly employed for conducting bulk analyses of larger portions of geological materials.
The method of analyzing major and trace elements in rocks, minerals, and sediment using X-ray spectrometers is highly prevalent due to its cost-effectiveness and straightforward sample preparation process. Additionally, the stability and user-friendly nature of X-ray spectrometers contribute to their widespread adoption in this field.
Interesting Science Videos
X-ray Fluorescence Spectrometry
- X-rays are a form of electromagnetic radiation that shares similarities with visible light rays, yet possesses an exceptionally short wavelength ranging from 100A to 0.1A.
- In contrast to conventional electromagnetic waves, X-rays exhibit a higher degree of penetrability through various substances, and their intensity is observed to increase inversely with the atomic number of the medium traversed.
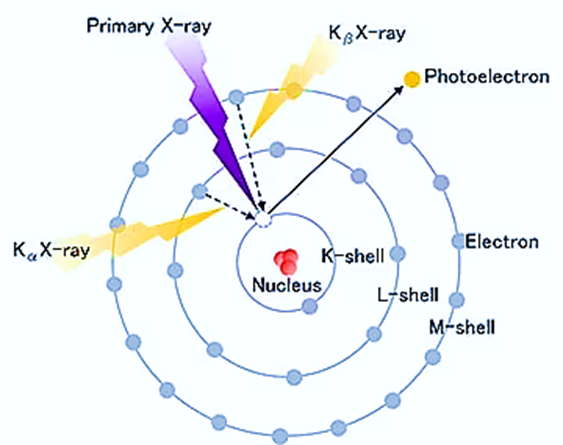
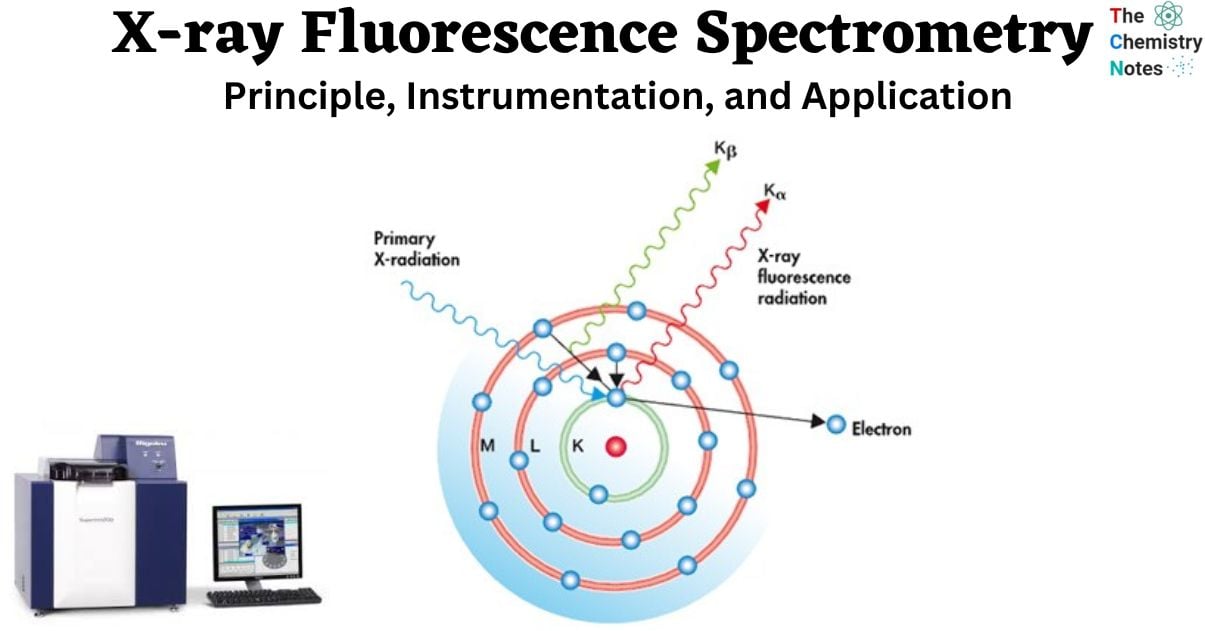
- X-ray fluorescence analysis is a technique that employs the emission of characteristic X-rays, known as fluorescent X-rays, resulting from the interaction between X-rays and a given material.
- Fluorescent X-rays are a type of electromagnetic radiation that is generated when X-rays, upon irradiation, induce the displacement of inner-shell electrons from their original positions to outer shells. Consequently, outer shell electrons swiftly transition to inner shells in order to occupy the previously vacant positions.
The energies of these fluorescent X-rays exhibit distinct characteristics for each element, allowing for qualitative analysis through the application of Moseley’s law. Additionally, quantitative analysis can be performed by assessing the intensity, or the number of photons, associated with each X-ray energy.
X-ray fluorescence analysis can be conceptualized as a form of spectrochemical analysis that operates within the X-ray region. The method shares similar attributes with atomic absorption spectrometry and optical emission spectrometry, with the exception that the sample under analysis does not require dissolution in a solution. Flameless atomic absorption spectrometry (FLAAS) involves the atomization of elements present in a sample within a flame operating at temperatures ranging from 2000 to 3000 degrees Celsius. ICP optical emission spectrometry (ICP-OES) involves the utilization of a plasma flame with temperatures ranging from 6000 to 9000C to induce excitation in a given sample. X-ray fluorescence also employs the process of sample excitation in order to extract information from X-rays.
X-ray Fluorescence Spectrometry Principle
The X-ray fluorescence (XRF) method relies on underlying principles that are employed by various instrumental methods that involve the interaction between electron beams, X-rays, and samples. These methods include X-ray spectroscopy (such as scanning electron microscopy with energy-dispersive X-ray spectroscopy), X-ray diffraction (XRD), and wavelength dispersive spectroscopy (such as microprobe wavelength dispersive spectroscopy).
- The ability to analyze major and trace elements in geological materials using X-ray fluorescence is facilitated by the atomic response to radiation.
- When subjected to high-energy, short-wavelength radiation such as X-rays, materials have the potential to undergo ionization.
- When the energy of the radiation is adequate to displace an inner electron that is tightly bound within an atom, the atom undergoes destabilization, leading to the substitution of the absent inner electron for an outer electron.
- The occurrence of this phenomenon results in the liberation of energy, which can be attributed to the diminished binding energy of the inner electron orbital in comparison to that of an outer electron orbital.
- The radiation that is released has lower energy compared to the initial X-rays and is referred to as fluorescent radiation.
- The detection of element abundances in a sample can be achieved by utilizing the characteristic energy of emitted photons, which corresponds to transitions between specific electron orbitals within the element.
- This phenomenon enables the utilization of fluorescent X-rays for this purpose.
X-ray Fluorescence Spectrometry Instrumentation
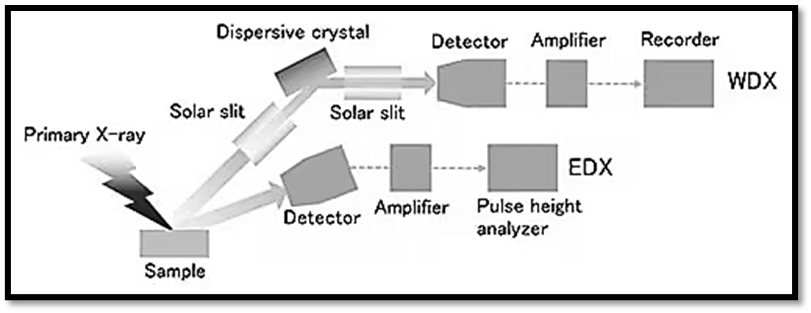
Analysis instruments for X-ray fluorescence can be roughly divided into two groups: those that use wavelength-dispersive X-ray spectroscopy (WDX) and those that use energy-dispersive X-ray spectroscopy (EDX). The huge size of the WDX equipment is due to the fact that fluorescent X-rays produced in a sample must be dispersed using an analyzing crystal and a goniometer. In contrast, the EDX detector offers better energy resolution and can function without a bulky dispersion system, resulting in a more compact instrument.
X-ray generation
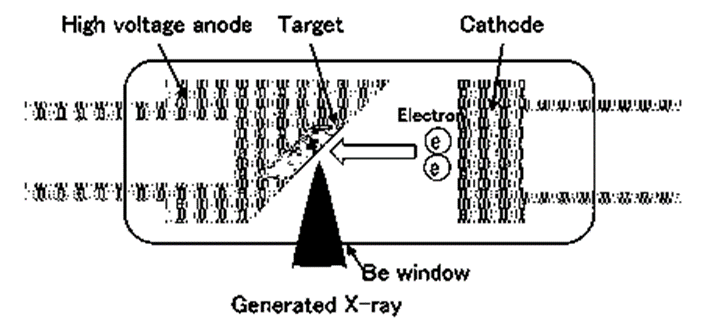
- The X-ray tube works by accelerating electrons at a high voltage and bombarding them against a metal anode (anti-cathode), producing X-rays in the process. Side window X-ray tubes and end window X-ray tubes are the two main varieties. Both are made to equally distribute high-powered X-rays throughout the surface of the sample.
- In most situations, a window made of beryllium foil is employed to collect incident X-rays. Anti-cathodes include elements including tungsten, rhodium, molybdenum, and chromium. The term target can also be used to refer to the anti-cathode. The type of samples to be tested dictates the anti-cathode to use. It is not a good idea to employ X-ray tubes that have anti-cathodes made of the same elements being studied.
Detector
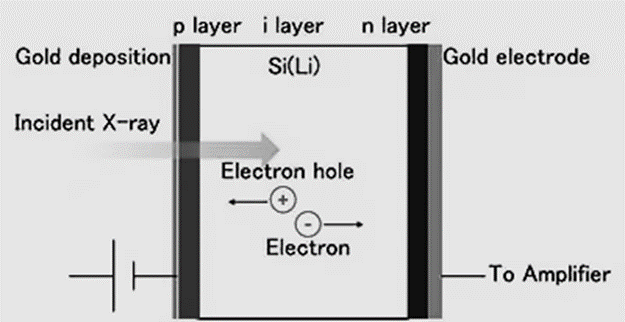
- The Si (Li) detector uses a diode with a p-i-n junction. The diode acts as a rectification mechanism, restricting electric current to flow in just one direction.
- The current is blocked when a voltage is given in the opposite direction of the electrons’ motion (reverse bias), but when light is allowed through, the electrons in the forbidden band are stimulated into a conductive band, and only the current for the excited electrons will flow.
- In order to detect X-rays, the individual current pulses that each incident X-ray photon generates must be measured.
- Since the incident X-ray energy is proportional to the instantaneous current value of a single pulse, the X-ray energy can be determined by measuring the pulse height of the current pulse.
- A diode with Li drifting across a high-purity single Si crystal, cooled by liquid nitrogen, is the Si (Li) semiconductor detector. Liquid nitrogen cools the FET.
- The first semiconductor detector was damaged by excessive voltage due to a liquid nitrogen shortage and rising temperature.
- Today’s detectors monitor their surface temperature and shut off the high voltage if it rises beyond a specified temperature to prevent inadvertent injury.
- At low frequencies, the detector can be utilized 30 minutes after liquid nitrogen.
Sample Chamber & Measurement Atmosphere
Both top-surface and bottom-surface sample chambers are used to irradiate samples with X-rays from different angles. Top-surface irradiation makes it simpler to observe samples and take measurements while moving around a stage, but both types can be detected.
The sample chamber environment can be decreased to vacuum conditions in the majority of X-ray fluorescence analysis devices. This is because X-rays are attenuated by the atmosphere, rendering them less effective, therefore measuring lighter materials necessitates a vacuum.
How Does X-ray Fluorescence Spectrometry Work?
The workings of an XRF spectrometer rely on the fact that when a sample is exposed to a strong X-ray beam (the incident beam), some of the energy is dispersed by scattering and some is absorbed by the sample itself in a way that is chemically dependent. In most cases, a Rh target is utilized to generate the incoming X-ray beam; however, other materials such as W, Mo, and Cr may be used.
The sample is said to be “excited” when it is exposed to this primary X-ray radiation. In response, the stimulated sample releases X-rays with a spectrum of wavelengths specific to the atom types present in the sample. What causes this to occur?
- Atoms in the sample ionize after absorbing X-rays, releasing electrons from their lower energy levels (often K and L).
- When electrons are evicted, higher energy electrons from a more distant orbital are sucked in to take their place.
- Since the binding energy of an inner electron orbital is less than that of an outer one, energy is liberated in this process.
- This heat is given off in the form of X-rays that are specific to each atom type. Using a Wavelength Dispersive Spectrometer similar to that found in an EPMA, one can dissect a complex emission X-ray spectrum into distinctive wavelengths for each element present in a sample with many elements present, as is usual for most minerals and rocks.
- The strength of the emitted beam is measured via gas flow proportional detectors and scintillation detectors, among others.
- The flow counter is frequently used to measure the K spectra of elements lighter than zinc, which typically emit X-rays with a long wavelength (>0.15 nm).
- Typically, the scintillation detector is employed to examine the X-ray spectrum at shorter wavelengths (K spectra of elements from Nb through I; L spectra of Th and U). When measuring intermediate-wavelength X-rays, both detectors are typically used together.
- This is because the K spectra created from Zn to Zr and the L spectra produced from Ba and the rare earth elements have different detection limits.
- These detectors assess energy levels that are proportional to the amount of elements in a given sample. Mineral or rock standards whose composition has been analyzed in the past provide the basis for deriving the exact value of this proportionality for each element.
Is X-ray fluorescence spectrometry safe?
When the tube in the analyzer is turned on, it releases a beam of radiation for use in the analysis. Every effort should be made to keep radiation doses as low as reasonably possible. The ALARA concept (As Low as Reasonably Achievable) states that this should be the goal. Time, distance, and shielding are three ways to reduce your exposure to radiation.
It is imperative to use caution when operating a portable or handheld XRF elemental analyzer, as the radiation emitted is comparable to the level of exposure encountered during routine medical or dental X-ray procedures. It is crucial to consistently direct the handheld XRF analyzer exclusively towards the sample being analyzed, and never towards an individual or any anatomical region of the body. Presented below are seven safety tips:
- It is essential to provide extensive radiation safety training to operators.
- It is imperative to stay away from controlling the device towards oneself or others while the primary beam, specifically when the x-ray is activated and the corresponding lights are illuminated.
- It is not advisable to retain samples during the process of analysis.
- It is important to utilize cautiousness and remain aware of the primary beam indicator lights.
- It is mandatory to handle and utilize with utmost respect.
- In the event of a safety emergency, it is imperative to promptly inform both the Radiation Safety Officer (RSO) and the vendor responsible for the analyzer.
Sample preparation for XRF
- If sufficient standards are accessible, it is possible to analyze nearly any solid or liquid substance.
- In the context of rocks and minerals, conventional instruments utilized in commercial settings typically necessitate a sample size consisting of several grams of material, although it is worth noting that the collected sample may potentially exceed this quantity.
- In the context of XRF chemical analyses of rocks, it is customary to collect samples that exceed the dimensions of the largest grain or particle present in the rock by several multiples.
- The initial sample undergoes a sequence of compressive procedures to achieve an average particle size ranging from a few millimeters to a centimeter.
- Subsequently, the sample can be further reduced through division into a smaller, representative subset weighing tens to hundreds of grams.
- The provided small sample is subsequently pulverized into a fine powder using various methods in order to generate the XRF sample.
- Special attention should be paid during this stage to ensure awareness of the composition of the crushing tools, as they will unavoidably introduce some level of contamination to the sample.
Interpretation of XRF spectra
The majority of atoms possess multiple electron orbitals, such as the K shell, L shell, and M shell. When X-ray energy induces electron transitions between different shell levels, it results in the generation of X-ray fluorescence (XRF) peaks with diverse intensities. These peaks are observable in the spectrum, which is a graphical depiction of the X-ray intensity peaks as a function of energy. The identification of an element is typically determined by the peak energy, while the concentration of the element is generally inferred from the height or intensity of the peak.
Advantages of X-ray Fluorescence Spectrometry
- The proposed methodology offers a streamlined, expeditious, and secure approach to sample preparation while mitigating the generation of chemical waste.
- It is a non-destructive analytical technique (a method that allows for the examination and analysis of materials without causing any damage or alteration to the sample being studied).
- Analysis at the production site
- Daily re-calibration is not required
- The affordability of owning
Limitations of X-ray Fluorescence Spectrometry
- In practical applications, the majority of commercially accessible instruments exhibit significant limitations in their capacity to accurately and precisely measure the elemental abundances of elements with atomic numbers less than 11 in various natural earth materials.
- X-ray fluorescence (XRF) analyses lack the capability to differentiate between isotopic variations within an element, necessitating the routine utilization of alternative instruments for such analyses.
- X-ray fluorescence (XRF) analyses are limited in their ability to differentiate between ions of the same element but in different valence states. Consequently, alternative techniques such as wet chemical analysis or Mossbauer spectroscopy are employed for the analysis of rocks and minerals.
Application of X-ray Fluorescence Spectrometry
- XRF finds significant relevance in various fields of research pertaining to igneous, sedimentary, and metamorphic petrology.
- Quality control in soil surveys
- Widely used in cement production, mining, environmental study, and the petroleum industry.
- Helps in the comprehensive chemical analyses of major elements (including Si, Ti, Al, Fe, Mn, Mg, Ca, Na, K, and P) in both rock and sediment samples.
- XRF focuses on conducting comprehensive chemical analyses of trace elements in rock and sediment samples. Specifically, we are interested in trace elements with abundances greater than 1 ppm, namely Ba, Ce, Co, Cr, Cu, Ga, La, Nb, Ni, Rb, Sc, Sr, Rh, U, V, Y, Zr, and Zn. It is worth noting that the detection limits for these trace elements are commonly in the range of a few parts per million.
Conclusion
Due to its non-destructive and expeditious nature, X-ray fluorescence analysis finds extensive application across various domains, including manufacturing and quality control. In recent times, the field of quantitative analysis of trace elements has witnessed significant advancements owing to the advent of high-sensitivity technologies. These technologies, including filtering techniques that effectively eliminate background interference and thin film methods, have made it feasible to conduct precise measurements and analyses of trace elements. X-ray fluorescence analysis is expected to experience increased prevalence in the future, specifically in the realm of assessing the presence of hazardous metals in various materials and soils.
References
- https://serc.carleton.edu/research_education/geochemsheets/techniques/XRF.html#:~:text=An%20X%2Dray%20fluorescence%20(XRF,an%20electron%20microprobe%20(EPMA).
- https://www.thermofisher.com/blog/ask-a-scientist/what-is-xrf-x-ray-fluorescence-and-how-does-it-work/
- https://www.hitachi-hightech.com/global/en/knowledge/analytical-systems/xrf/xrf-descriptions.html
- https://www.xrfscientific.com/working-principle-xrf-spectrometer/