Bohrium is a synthetic transition metal with an atomic number of 107 and is represented by the symbol ‘Bh’ in the periodic table. It is silvery in appearance and belongs to the d-block of period 7 of the periodic table. Bohrium was the fourth transactinide (super-heavy) element identified. Only tiny quantities of bohrium have been successfully synthesized, hence there isn’t much known about it based on experimental data, but some qualities can be predicted using periodic table trends.
Peter Armbruster, Gottfried Münzenberg, and their German team at the GSI Helmholtz Centre or Heavy Ion Research in Darmstadt are credited for discovering and isolating element 107.
Interesting Science Videos
Discovery and History of Bohrium
- Bohrium was initially produced as isotope-261 at the Russian Joint Institute for Nuclear Research in 1975. The research team that was led by Yuri Oganessian, published their findings in 1976.
- Yuri Oganessian and his team targeted bismuth-209 and lead-208 with chromium (Cr)-54 and manganese-58 nuclei, respectively.
- The team thought they had obtained bohrium-261 and dubnium-258, which decay into bohrium-262.
- However, Peter Armbruster, Gottfried Münzenberg, and their German colleagues at the GSI Helmholtz Centre or Heavy Ion Research in Darmstadt are credited with discovering and isolating element 107.
- In 1981, they used chromium-54 nuclei to blast a bismuth-209 target, resulting in 5 atoms of bohrium-262.
- In honor of physicist Niel Bohr, a German team proposed the element name nielsbohrium and the symbol Ns.
- Russian scientists from the Joint Institute for Nuclear Research in Dubna, Russia, proposed the element name for element 105.
- Finally, 105 was named dubnium, and the Russian team agreed to the German proposal for element 107.
Occurrence of Bohrium
- Bohrium is not found naturally in the Earth’s crust; it must be synthesized in particle accelerators. It cannot even be manufactured in a nuclear reactor.
- Bohrium does not exist in nature. It is synthetically produced in limited quantities.
- It is produced artificially by blasting bismuth-204 with chromium-54. The reaction yielded five atoms of bohrium.
- It is created synthetically using cold fusion.
- Bohrium contains eleven isotopes with known half-lives: 260Bh, 261Bh, 262Bh, 264Bh, 265Bh, 266Bh, 267Bh, 270Bh, 271Bh, 272B, and 274Bh.
Elemental Properties of Bohrium
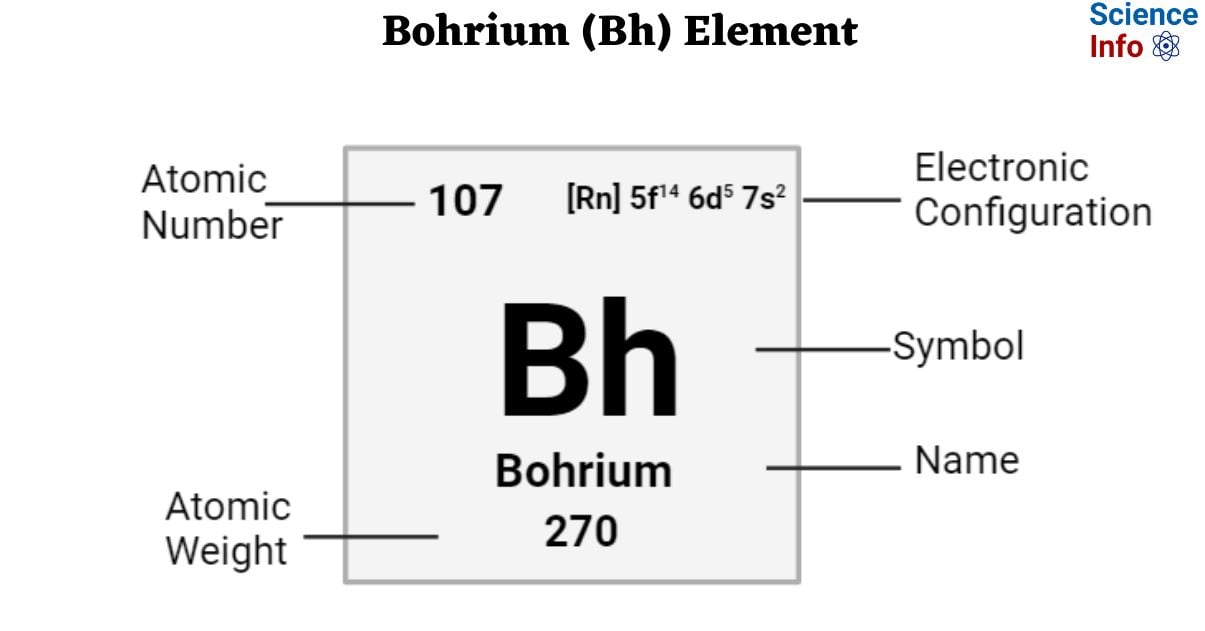
| Electronic Configuration | [Rn] 5f14 6d5 7s2 |
| Atomic Number | 107 |
| Atomic Weight | 270 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Trans-actinides, 7, d-block |
| Density | 37.1 g/cm3 |
| Ionic radius | – |
| Van der Waals radius | – |
| Electron shells | 2, 8, 18, 32, 32, 13, 2 |
| Electrons | 107 |
| Protons | 107 |
| Neutrons | 163 |
Isotopic Information of Bohrium
- Bohrium has no stable isotopes naturally, but they can be created in a laboratory setting.
- All of the bohrium isotope are unstable and radioactive.
- Bohrium contains eleven isotopes with known half-lives: 260Bh, 261Bh, 262Bh, 264Bh, 265Bh, 266Bh, 267Bh, 270Bh, 271Bh, 272B, and 274Bh.
- At least one metastable state exists. Isotopes decay by alpha decay. Other isotopes could be prone to spontaneous fission.
- Lighter isotopes, such as 260Bh, 261Bh, and 262Bh, have half-lives less than 100 milliseconds.
- The half-lives of 264Bh, 265Bh, 266Bh, and 271Bh are around 1 second, whereas those of 267Bh and 272Bh are about 10 seconds.
- 270Bh and 274Bh have 61 seconds and 40 seconds, respectively, while 278Bh has a longer half-life of around 10 minutes.
Physical Properties of Bohrium
- The instability of bohrium makes it difficult to conduct a statistically significant investigation of its physical properties.
- Due to its rapid disintegration, only a few properties of bohrium have been investigated until now.
- Bohrium is a synthetic, super-heavy transactinide element. It is expected to be solid under normal conditions.
- It is found in the 7th period, the 7th Group, and the d-block of the periodic table.
- The melting point and the boiling point of the element 107 is yet to be known.
- The atomic mass of bohrium is 270.
- The density of bohrium is also unknown as of now.
- It is malleable and can conduct both heat and electricity.
- Bohrium is a heavy metal with a density of 37.1 g/cm3, ranking third among the 118 known elements of the periodic table.
- It is the heaviest member of group 7.
Chemical Properties of Bohrium
- Radiation-emitting bohrium shares many chemical characteristics with the group 7 elements and therefore behaves as a heavier homologue (a set of compounds distinguished from one another by repeating units) with rhenium.
- The features of bohrium that have been investigated are solely connected to singular chemistry. The majority of the attributes are purely theoretically determined.
- Bohrium exists in a +7 oxidation state, although it also exhibits lower states such as +3 and +4, which technetium and rhenium do.
- In an aqueous solution, bohrium (VII) is volatile and readily converted to a more stable bohrium (IV).
- Bohrium is known for generating a range of oxides and oxychlorides.
- It can also generate the volatile oxides Bh2O7.
- These oxides dissolve in water, forming pyroboric acid (HBhO4).
Synthesis of Bohrium
- All elements with atomic numbers more than 100 can only be created through reactions in a particle accelerator, such as a cyclotron; they do not develop in a nuclear reactor.
- Bismuth-209 blasted with chromium-54 produces bohrium-262.
Uses of Bohrium
- Given barely any atoms of this metal have been created to date, there are currently no specific or exclusive uses of Bohrium outside of scientific research.
- Furthermore, because it is unavailable in nature, Bohrium is only employed by scientific researchers, with no recognized negative effects or uses for the metal among individuals and organizations.
- Bohrium is currently solely used in studies to learn more about its characteristics and to manufacture isotopes of other elements.
- Bohrium performs no biological purpose. Because it is a heavy metal that decays to release alpha particles, it is highly poisonous.
Health Effects of Bohrium
- Bohrium is a very unstable chemical; when created, it swiftly decomposes into other elements, therefore it has no influence on human health.
Environmental Effects of Bohrium
- Bohrium’s environmental effects are negligible due to its short half-life (just a few minutes).
Video Reference
References
- https://www.thoughtco.com/bohrium-facts-element-107-or-bh-4125948
- https://www.utoledo.edu/nsm/ic/elements/bohrium.html
- https://periodic-table.com/bohrium/
- https://www.chemicool.com/elements/bohrium.html
- https://chemicalengineeringworld.com/bohrium-element-properties-and-information/

