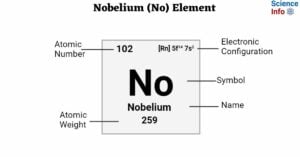
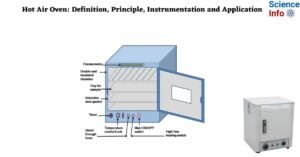
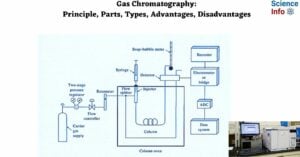
Hydrodynamic Chromatography: Definition, Principle, and Applications
Hydrodynamic chromatography (HDC) is a technique used for separating particles based on their size, enabling the measurement of particle size distributions within the 20 – 1,000 nm range. This method expands the capability of traditional size exclusion chromatography to handle … Read more