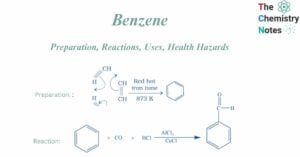
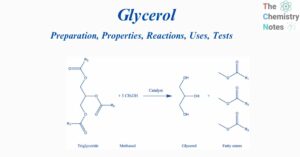
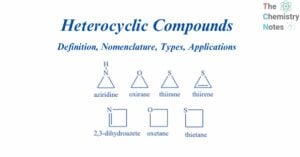
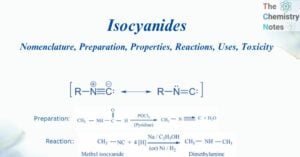
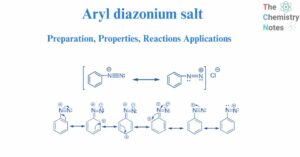
Benzene: Preparation, Reactions, Uses, Health Hazards
Benzene, an aromatic hydrocarbon, having the chemical formula C6H6, is one of the most significant organic molecules. It is the primary component of all aromatic compounds. At room temperature, It is a colorless or light-yellow liquid compound. It is largely … Read more