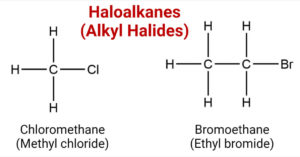
Haloalkanes (Alkyl Halides)- Structure, Preparation, Properties, Uses
Haloalkanes are the organic compounds containing the halogen group attached to the carbon. The halogen bonded to the carbon atom acts as a functional group of haloalkane. eg: They have the general formula R-X. where R= alkyl group, X= Cl, … Read more