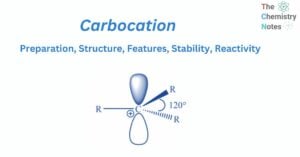
Carbocation: Preparation, Structure, Features, Stability, Reactivity
A carbocation is a name used to describe organic chemical entities with an electrical charge on a carbon atom. The carbocation has a positively charged carbon. In general, these carbon atoms react with various elements to provide a reactant for … Read more