Cesium (Cs) Element: Properties, Reactions, Uses, Effects
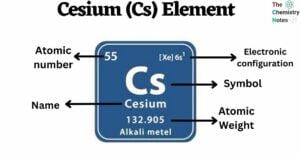
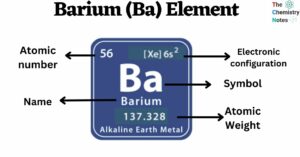
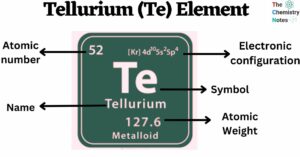
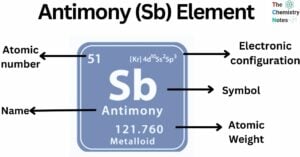
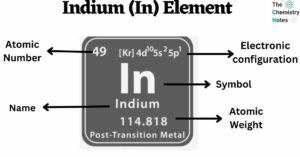
Cesium is a chemical element with the atomic number 55 and is represented by the symbol ‘Cs’ in the periodic table. It is a soft, silvery-golden alkali metal and belongs to the s-block of group 1 of the periodic table. … Read more