Lawrencium is a synthetic chemical element with an atomic number of 103 and is represented by the symbol ‘Lr’ in the periodic table. It is silvery in appearance and belongs to the f-block of period 7 of the periodic table. Lawrencium was identified as the last synthetic trans-uranium element in the actinide series. Similar to other elements in the actinide class, lawrencium exhibits significant radioactivity.
Lawrencium was produced independently by a team of scientists from the Joint Institute for Nuclear Research (JINR) in Dubna and the American Lawrence Berkeley Laboratory. In 1961, a team of physicists working at Berkeley, Almon Larsh, Torbjorn Sikkeland, Albert Ghiorso, and Robert Latimer synthesized an atom of lawrencium by blasting a californium target with boron nuclides.
Interesting Science Videos
Discovery of Lawrencium
- Lawrencium was produced independently by a team of scientists from the Joint Institute for Nuclear Research (JINR) in Dubna and the American Lawrence Berkeley Laboratory.
- Almon Larsh, Torbjorn Sikkeland, Albert Ghiorso, and Robert Latimer, physicists at Berkeley, synthesized an atom of lawrencium in 1961 by blasting a californium target with boron nuclides.
- Working at the Lawrence Radiation Laboratory in Berkeley, California, the scientists added three micrograms (0.000003 grams) of californium to the target chamber of a linear accelerator.
- Later, in 1965, a team of scientists from Dubna successfully confirmed the creation of lawrencium.
- The International Union of Pure and Applied Chemistry (IUPAC) honored both teams for finding lawrencium in 1992.
- The name lawrencium was officially announced in 1997 and is named after Ernest Lawrence, who invented the cyclotron, which is used to detect radioactive elements.
Occurrence of Lawrencium
- Lawrencium is a synthetic element that does not occur naturally.
- Lawrencium is formed through nuclear bombardment and has only been produced in trace amounts.
- The irradiation of californium-249 with boron-11 ions can produce lawrencium-256.
- Irradiating berkelium-249 with oxygen(O)-18 ions can produce lawrencium-260.
- Lawrencium contains ten isotopes having known half-lives, ranging from mass numbers 253 to 262.
- The element 103 does not have any naturally occurring isotopes.
Elemental Properties of Lawrencium
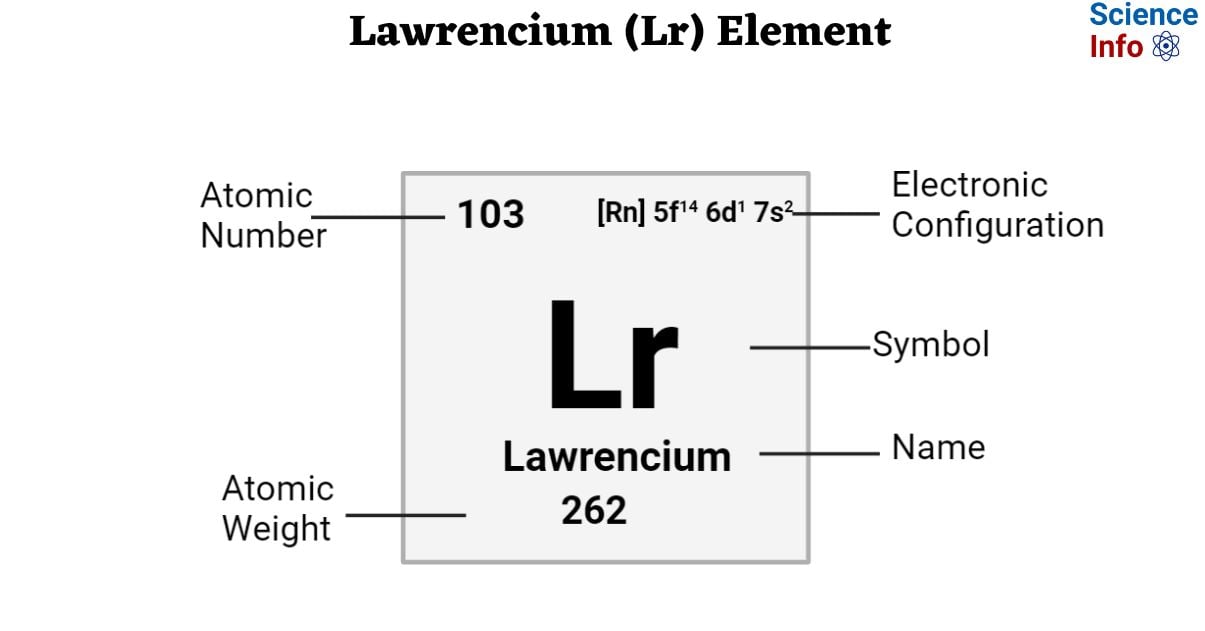
| Electronic Configuration | [Rn] 5f14 6d1 7s2 |
| Atomic Number | 103 |
| Atomic Weight | 262 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Actinide, 7, f-block |
| Density | 16 g/cm3 at 20 °C |
| Ionic radius | unknown |
| Van der Waals radius | – |
| Electron shells | 2, 8, 18, 32, 32, 9, 2 |
| Electrons | 103 |
| Protons | 103 |
| Neutrons | 159 (Varies with isotopes) |
Isotopic Information of Lawrencium
- Lawrencium contains ten isotopes with known half-lives, ranging in mass from 253 to 262. It contains no naturally occurring isotopes.
- The most stable isotope is lawrencium-266, which has a half-life of 11 hours.
- Lawrenium-260 has a half-life of around three minutes and is commonly utilized in laboratory research due to its ease of production.
- Most lawrencium isotopes decay by emitting alpha particles.
- The lightest isotopes of lawrencium, lawrencium-251 to lawrencium-254, are produced as alpha decay products of dubnium.
Physical Properties of Lawrencium
- Because of its unstable nature, lawrencium’s physical characteristics are challenging to analyze quantitatively.
- Atomic mass of the element 103 is 262 u.
- The final element in the actinide series is lawrencium. On the periodic table, it lies to the left of rutherfordium and to the right of nobelium.
- It is silvery in appearance.
- The density of lawrencium is 16gm/cm3.
- It is the heaviest element among the actinide series.
- It has the melting point of 1627 degree Celsius, whereas boiling point is yet to be known.
- Lawrencium is thought to have several physical similarities with lutetium. Its atomic volume is comparable to that of lutetium.
- Lawrencium is expected to be solid at typical temperatures and pressures.
- It is projected to have a hexagonally closed-packed structure, similar to lutetium.
- Lawrencium’s electronic configuration is anomalous.
Chemical Properties of Lawrencium
- Lawrencium’s unstable nature makes it difficult to conduct statistically significant analyses of its chemical properties.
- The element 103 is expected to share the chemical properties similar to that of lutetium.
- It has a comparable enthalpy of sublimation to lutetium.
- The most common oxidation state for lawrencium is projected to be +3.
- Lawrencium metal is anticipated to be readily oxidized by oxygen, steam, and acids.
- The stability of lawrencium(III) compounds in aqueous solution is comparable to that of lutetium(III).
- Element 103 is believed to generate chloride, fluoride, hydroxide, and hydride.
Synthesis of Lawrencium
- All elements with atomic numbers more than 100 can only be created through reactions in a particle accelerator, such as a cyclotron; they are not generated in a nuclear reactor.
- The alpha decay products of dubnium generate the lightest isotopes of lawrencium, which are lawrencium-251 to lawrencium-254.
- In the same way as lighter lawrencium isotopes, the heaviest lawrencium isotopes: lawrencium-264 to lawrencium-266 are synthesized.
- After subjecting actinide atoms ranging from americium to einsteinium to light ions ranging from boron to neon, the middle isotopes of lawrencium, lawrencium-255 to lawrencium-262, are created.
Uses of Lawrencium
- Lawrencium is not very useful because of its radioactive nature. Lawrencium is solely utilized in scientific investigations.
- Lawrencium is not used commercially because just a few atoms have been produced.
- This element has technical applications as well as energy harvesting capabilities.
- 260-Lr is the widely utilized lawrencium in chemistry lab for research purpose due to its greater production.
Health Effects of Lawrencium
- Lawrencium doesn’t exist naturally, has not yet been discovered in the earth’s crust, and is so unstable that any amount generated would rapidly dissolve into other elements. As a result, there is no reason to worry about its potential health risks.
Environmental Effects of Lawrencium
- Lawrencium’s short half-life indicates that its effects on the environment are not worth taking into account.
Video Reference
References
- https://www.periodic-table.org/lawrencium-periodic-table/
- https://www.lenntech.com/periodic/elements/lr.htm
- https://periodic-table.com/lawrencium/
- https://chemicalengineeringworld.com/lawrencium-element-properties-and-information/
- https://www.chemicool.com/elements/lawrencium.html
- https://www.vedantu.com/chemistry/lawrencium

