Hybridization: Definition, Features, Types
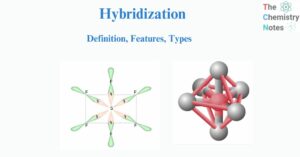
Hybridization is the process of combining two or more non-equivalent atomic orbitals with similar energies but different forms. The process of combining two atomic orbitals to form a new type of hybridized orbitals is termed hybridization. Atomic orbitals of the same … Read more