Neon (Ne) Element: Properties, Uses, 10 Incredible Facts
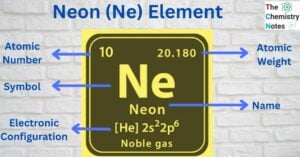
Neon (atomic number 10, symbol Ne) is an inert noble gas and a chemical element. The name neon derives from the Greek word νέoν, the neuter singular form of νέος (neos), meaning ‘new’. Neon is chemically inert, and there are no … Read more