A property of a metal to withstand being pulled or stretched before fracturing is known as ductility. It is among the most important mechanical characteristics of a metal. Businesses would not be able to ensure the soundness of their appliances without a solid understanding of the science of ductility. It is a characteristic mostly found in metals and is based on the quantity and arrangement of electrons inside the atom.
Interesting Science Videos
What is Ductility?
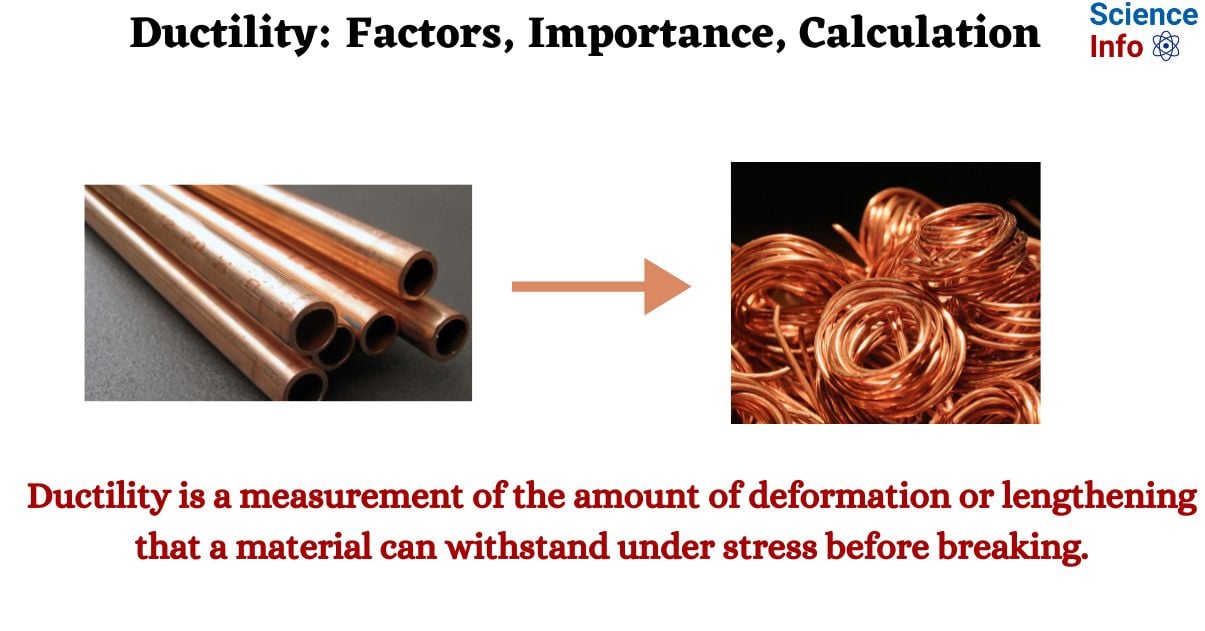
The ability of a material to be stretched, pulled, or dragged into a thin wire or thread without breaking is known as its ductility. It is a measurement of the amount of deformation or lengthening that a material can withstand under stress before breaking. Generally speaking, ductility is mostly connected to metals. In metals, bonds known as metallic bonds form between atoms. This only suggests that electrons can freely move between any two atoms. The atoms in the metal can also glide past one another thanks to this property, which permits the metal to be stretched.
The number as well as the arrangement of electrons within an atom determine a metal’s ductility. Electrons are grouped in rings around the nucleus of an atom; these rings are called electron shells. The electrons that travel within a metal and are found in its outermost shell are known as its valence electrons. Generally speaking, metals having the highest concentrations of electron shells and outer shell electrons are the most ductile. As a metal’s temperature rises, its valence electrons become more mobile, increasing the metal’s ductility.
Science of Ductility
- One of the most important characteristics of metal is the metallic bond, in a ductile metal an electron from some atoms separates and travels freely throughout the entire material field.
- This movement produces a unique environment in the metal’s structure known as a slip plane.’ Slip planes allow areas densely packed with atoms to pass each other without collision.
- On a molecular level, the atoms simply slip by each other and carry on their route rather than meeting and causing a fracture.
- With a few notable exceptions, most ductile metals contain more electron shells, making it simple to identify them from the less ductile metals from this atomic perspective.
- The orbits that electrons follow around the nucleus of an atom are known as electron shells. The physical maximum number of electrons that each shell may hold is known.
- After a shell is filled, electrons form and continue another orbit outside of the filled shell to locate space.
- In this regard, a metal’s ductility and total electron count are related to one another.
Factors That Determine The Ductility
The ductility of a metal is determined by different factors which are discussed here:
Impurities
Impurities that are present in an element could affect its ductility. Impurities weaken atomic bonds, making it more difficult for the dislocation to migrate and ultimately decreasing the material’s ductility. Therefore, removing all impurities is critical for increasing ductility.
Crystalline Structure
The material’s ductility is also affected by its crystalline structure. The crystalline structure of materials, such as metals, can vary based on their composition, which can have an impact on their ductility. Ductility is often higher for materials with a face-centered cubic crystalline structure compared to those with a body-centered cubic crystalline structure or hexagonal close-packed crystalline structure. For instance, metals with hexagonal crystalline structures, such as titanium, become less ductile compared to those with cubic crystalline structures, such as copper and aluminum.
Grain Size
The material’s ductility is additionally affected by the grain’s size. Metals with smaller grains are more ductile because the grain boundaries function as barriers to displacement slide. Smaller grain sizes result in higher boundaries among grains along with lower ductility, but higher strength and hardness. Larger grain sizes result in lesser boundaries between grains and higher ductility, however with a loss of hardness and strength.
Temperature
Temperature influences a material’s ductility. Materials stiffen and become less ductile at low temperatures, whereas they soften and become more ductile at high temperatures. Cold working metal reduces ductility by deforming the extremely small grain structure of the metal, which increases internal stresses. This reduces ductility as well as increases hardness and strength. Under sufficiently extreme temperatures, the interior grain structure of a metal transforms to the extent that a great deal of the internal stress gets eliminated. This results in increased ductility but lower strength and hardness.
Calculating Ductility
Measuring ductility is crucial for all metals as it determines the appropriate shaping and machining procedures. It decides what kinds of uses the raw materials are best suited for. It can also help to identify the optimum load of the material.
While calculating the ductility you must keep these things in your mind.
Elongation
Elongation is an increase in the gauge length of a material exposed to tensile pressures divided by its initial gauge length. A percentage of elongation isn’t a precise metric in itself. The elongation is not entirely uniform and will be most severe in the center of the body. Elongation is commonly represented as a percentage of the initial gauge length.
Formula to Calculate The Elongation
Elongation (%) = ∆L/Lo x 100
Where,
∆L is the length shift that occurs after the material is subjected to tensile stress and fractures.
Lo is the initial gauge length.Reduction of Area
The second metric is the reduction in cross-sectional area. This is also stated in percentages. The reduction in area at the minimal diameter is a better indicator of ductility.
Formula to Calculate The Reduction of Cross-Sectional Area
Reduction of the C.A area (%) = (Ao – Af)/Ao x 100
Where,
Ao is the initial cross-sectional area.
Af is the cross sectional area after the fracture. It ought to represent the most narrow section of the material.Both of these statistics, presented as percentages, represent the ductility of the material under test. When performing the tensile stress test, keep in mind that temperature has a considerable influence on the ductility of the tested metal.
Testing The Ductility
Ductility testing, also known as bent testing, requires applying force to a specimen and documenting the degree at which the material begins to plastically deform or fracture. A basic test involves placing the specimen between two anvils and applying force with a machine.
The testing process for ductility is a useful and practical method to assess a specimen’s capability of deformation due to plasticity before breaking. It can be used with a variety of materials to determine where they bend or fracture. Essentially, it is a measure of how far a sample can be stretched without breaking. In metals, for example, the fracture forms a classic cone and cup shape, and the fracture surface appears rough and fibrous. Ductile materials exhibit a certain amount of plastic deformation before breakage.
Factors Affecting The Testing
Testing of ductility can be affected by these factors:
Dimension
The elongation measurements for an object are directly affected by its cross-sectional area. For the test to produce accurate outcomes, the specimen’s dimension must be maintained constant.
Gauge Length
While carrying out a tensile stress test, gauge length is an important metric to pay emphasis to. However, the value of elongation is less reliant on gauge length as gage length increases.
Strain Rate (Test Speed)
A greater or quicker stress reduces ductility, lowering the elongation value. Brittle materials tend to be more vulnerable to strain rate, and elongation values fall as the stress rate rises uniformly.
Some of The Most Ductile Metals
Here are some examples of the most ductile materials to exist.
Silver
Silver (Ag) is one of the most ductile metals on earth. It can be stretched into long thin wires without any fracture. Silver is mostly used in the jewelry, electronic products, automobile industries, and in brazing and soldering.
Gold
Gold (Au) is also an extremely ductile metal. One gram of gold is capable of being woven into a thread that is 2.4 kilometers (1.5 miles) long. It is generally employed in the making of jewelry, embroidery, and also in dentistry.
Copper
Copper is another ductile metal that is highly utilized in different industrial applications. More than half of the copper mined worldwide is used in the production of electrical wire as it is also the good conductor of electricity.
Aluminum
Aluminum (Al) is another example of ductile metal. It is also capable of being drawn into thin wires without breaking. It is highly utilized as an alloy for this very property.
Importance of Ductility in Our Life
As we know material’s ductility is a physical quality that determines how much it can flex without breaking. This property of metal allows certain areas to be strengthen. The major importance of ductility are discussed here:
Construction and Engineering
To withstand the applied loads and stresses, structural materials like steel and aluminum need to be extremely ductile. These materials’ ductility permits them to reposition stresses and undergo deformation, therefore minimizing structural collapse. Such materials’ ductility also makes them simpler to form and weld, increasing their versatility and ease of use. This can be helpful for various industrial appliances and for the safety of structures during earthquakes.
Automobiles Industry
Ductility also becomes important in automotive manufacturing. Automobile bodywork and parts must be made from highly ductile materials to absorb impact forces in the case of an accident. This protects car occupants by equally spreading impact energy.
Aerospace Industries
The aviation sector requires construction materials that can withstand severe pressure, stress, and unfavorable environmental conditions. Ductility helps these devices resist the severe nature of flying by maintaining the aircraft’s safety and reliability.
Household Appliances
Besides being beneficial to its industrial applications, ductility is crucial in the production of daily products. Electrical wires, for example, are comprised of highly ductile materials like copper, which allows for flexibility and ease of installation. These wires are used in the range of electrical gadgets we use in our daily lives. Similarly, plastic pipes used in plumbing are extremely ductile, making them easier to install in confined locations.
Jewelry
Some of the most ductile metals such as Gold, Silver, Platinum, and Aluminum are used to make jewelry. Because of the ductility, it is easier to work with these metals on a complicated design of jewelry. For instance, one gram of gold is capable of being woven into a thread that is 2.4 kilometers (1.5 miles) long.
Difference Between Ductility and Malleability
Ductility and malleability are both mechanical properties of metals, although they represent different elements of how metals react to external forces.
Ductility:
- Ductility refers to a metal’s ability to undergo significant deformation before fracturing or breaking.
- This deformation typically involves stretching or elongating the material.
- Metals with high ductility can be pulled into thin wires without breaking.
- Ductility is frequently related with the capacity of metal atoms to glide past one another, a phenomenon known as plastic deformation.
- This feature is critical in applications where metals must be stretched or shaped into diverse shapes without breaking, such as in wire drawing, tubing, or machining processes.
Malleability:
- Malleability, on the other hand, is the ability of a metal to withstand compression and deformation under pressure.
- It refers to the metal’s ability to be shaped or formed through hammering, rolling, or pressing without cracking or fracturing.
- Malleability enables metals to be flattened into thin sheets or molded into various shapes without breaking apart.
- Malleability, as opposed to ductility, which largely entails stretching or elongating the metal, is the rearranging of atoms within the material to accommodate the given pressure or force.
In summary, while both ductility and malleability require metal deformation, they reflect distinct forms of deformation: ductility refers to stretching or elongating the material, whereas malleability refers to compressing or shaping the material. Furthermore, metals with high ductility may not be highly malleable, and vice versa, depending on their crystal structure and bonding properties.
Frequently Asked Questions (FAQs)
What Is the Most Ductile Metal?
Gold, platinum, and silver are the natural metals with the highest ductility. All of these can be stretched into fine wires and used as jewelry. Copper is another exceptionally ductile metal that is often utilized in electrical cables.
Is ductility an extensive or intensive property?
Ductility is considered an Intensive property. The intensive property is independent of the amount of substances. Other examples of intense properties are color, melting point, odor, hardness, density, pressure, and so on.
What Does “High Ductility” Mean?
A material with high ductility is more likely to distort than break. A material with high strength and ductility will be more durable than a material with low strength and ductility. Brittle materials have constrained strain values, thus while they are strong, they are not tough
What Does “Low Ductility” Mean?
When subjected to tensile tension, a material with limited ductility would break rather than deform
Video Reference
References
- https://www.ametektest.com/learningzone/testtypes/ductility-testing
- https://polaridad.es/en/para-que-sirve-la-ductilidad/?expand_article=1
- https://www.thyssenkrupp-materials.co.uk/technical-knowledge-hub/how-to-calculate-ductility
- https://blog.eaglegroupmanufacturers.com/metal-properties-ductility
- https://efficientengineer.com/material-strength-ductility-toughness/
- https://www.corrosionpedia.com/definition/422/ductility
- https://www.xometry.com/resources/3d-printing/ductility/

