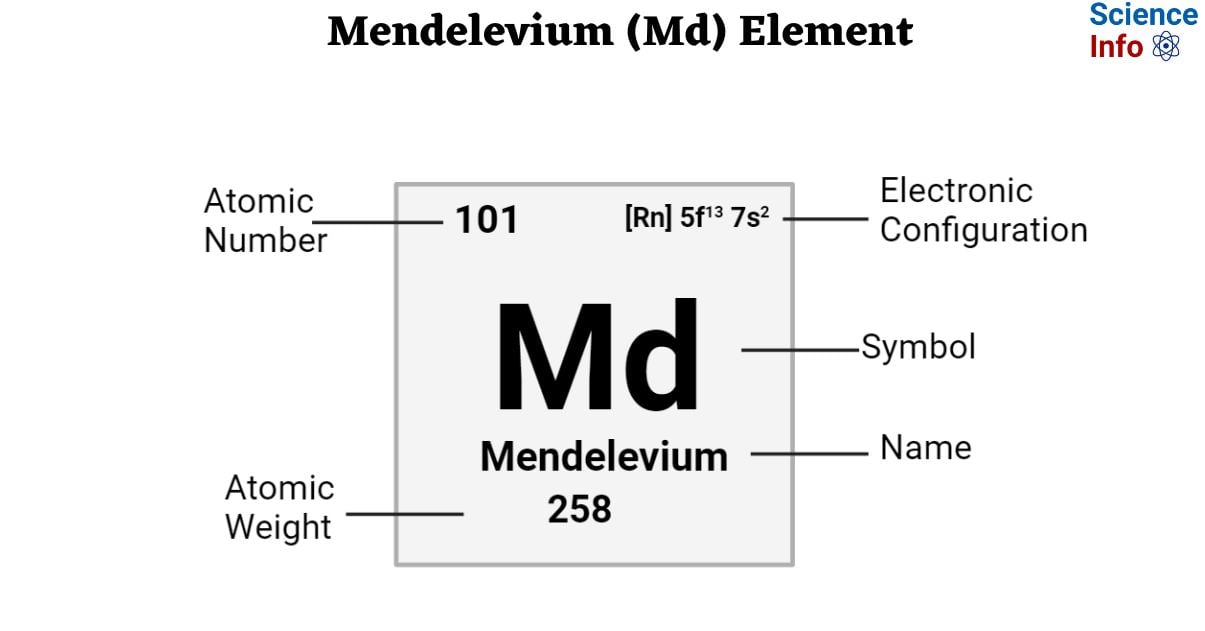
Mendelevium is a synthetic chemical element with an atomic number of 101 and is represented by the symbol ‘Md’ in the periodic table. It is silvery in appearance and belongs to the f-block of period 7 of the periodic table.
Mendelevium was identified as the ninth synthetic trans-uranium element in the actinide series. Similar to other elements in the actinide class, mendelevium exhibits significant radioactivity. It was discovered by a group of scientists Albert Ghiorso, Bernard Harvey, Gregory Choppin, Stanley Thompson, and Glenn Seaborg. At Berkeley, California, they produced Mendelevium-256 (with a half-life of 78.1 minutes) by bombarding Einsteinium-253 with helium ions in a 152-cm (60-inch) cyclotron. They then separated the element from other elements using chromatography.
Interesting Science Videos
Discovery of Mendelevium
- Mendelevium was synthesized by Stanley Thompson, Jr., Albert Ghiorso, Bernhard Harvey, Gregory Choppin and Glenn Seaborg.
- They produced Mendelevium-256 (with a half-life of 78.1 minutes) by bombarding Einsteinium-253 with helium ions in a 152-cm (60-inch) cyclotron. They then separated the element from other elements using chromatography.
- While one or two atoms should have been formed every three hours, mendelevium-256 was created during an all-night experiment. But just seventeen atoms were created. A neutron and mendelevium-256 were produced in each nuclear process.
- It was a first element to be produced one atom at a time.
- Element is named after Dmitri Mendeleev, a Russian chemist who created one of the first periodic tables.
- The proposed element symbol was Mv, but the IUPAC altered it to Md during their 1957 meeting in Paris.
Occurrence of Mendelevium
- Mendelevium is a synthetic element that does not occur naturally.
- It is created by charged particles of lighter elements in particle accelerators.
- Mendelevium is synthesized by striking bismuth (Bi) targets with argon (Ar) ions, plutonium (Pu) or americium (Am) targets with carbon (C) or nitrogen ions, and einsteinium with alpha particles.
- Mendelevium isotopes are all extremely unstable, decaying into Einsteinium through alpha decay or spontaneous fission.
- Mendelevium contains 16 identified isotopes. The mass number of these isotopes spans between 245 and 260.
Elemental Properties of Mendelevium
| Electronic Configuration | [Rn] 5f13 7s2 |
| Atomic Number | 101 |
| Atomic Weight | 258 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Actinides, 7, f-block |
| Density | 10.3 g/cm3 at 20 °C (not confirmed) |
| Ionic radius | unknown |
| Van der Waals radius | – |
| Electron shells | 2, 8, 18, 32, 31, 8, 2 |
| Electrons | 101 |
| Protons | 101 |
| Neutrons | 157 (Varies with isotopes) |
Isotopic Information of Mendelevium
- Mendelevium isotopes are radioactive and highly unstable, degrading into Einsteinium by alpha decay or spontaneous fission.
- Mendelevium contains 16 identified isotopes. The mass number of these isotopes spans between 245 and 260.
- The element has five nuclear isotopes that are known.
- Mendelevium-256 is the most widely utilized isotope for experiments due to its simplicity of synthesis and half-life of 1.17 hours.
- The isotopes with the most extended half-lives are 258-Md, (with the half-life of 51.5 days), 260-Md, (with the half-life of 31.8 days), and 259-Md, (with the half-life of 96 minutes).
Physical Properties of Mendelevium
- Since mendelevium has not yet been synthesized in large enough quantities, little is known about its physical characteristics.
- Mendelevium belongs to the actinide series and has an atomic number of 101.
- It is symbolized by the symbol Md.
- It is categorized as a metal and remains solid at normal temperature.
- Mendelevium’s atomic mass is 258.
- Mendelevium’s melting point is estimated to be 827°C. It’s boiling point is yet to be confirmed.
- Mendelevium is expected to have a density of approximately 10300 in SI units at 20°C.
- Theoretical features such as atomic radius have been proven through trace amount experiments.
- It’s ionic radius is less than that of the actinide that came before it in the periodic table, fermium.
- The density, state of matter, crystal structure, and melting point have been calculated using the behavior of neighboring elements on the table.
- Mendelevium is assumed to have a face-centered cubic crystal structure.
Chemical Properties of Mendelevium
- The element can produce trivalent (+3) and divalent (+2) ions. These oxidation states have been observed experimentally in solution. The +1 state has also been recorded.
- It is trivalent in nature in aqueous solution.
- Mendelevium creates coordination compounds with DCTA.
- Mendelevium (III) is readily reduced to mendelevium (II). When dissolved in water, it behaves steadily.
- Reduction causes it to act like a divalent element.
- It is neutral in water-ethanol solution and has properties similar to cesium (Cs).
- It produces insoluble fluorides and hydroxides, which are condensed into trivalent lanthanide salts.
- A carbon-exchange bond causes the mendelevium to elute.
- Mendelevium co-crystallizes with chlorides to generate mixed crystals containing divalent elements.
Production of Mendelevium
- All elements with atomic numbers more than 100 can only be created through reactions in a particle accelerator, such as a cyclotron; they cannot be produced in a nuclear reactor.
- The most important mendelevium isotopes, mendelevium-254 to mendelevium-256, are created by bombarding einsteinium-253, 254, and 255 with alpha particles.
- The lightest mendelevium isotopes, mendelevium-244 to mendelevium-247, are generated by bombarding bismuth targets with heavy argon ions.
- The somewhat heavier mendelevium isotopes, mendelevium-248 to mendelevium-253, are generated by bombarding plutonium and americium targets with lighter carbon and nitrogen ions.
- Mendelevium-260 is formed through a transfer reaction between einsteinium-254 and oxygen-18.
Uses of Mendelevium
- Because very limited amounts of mendelevium can be created and its isotopes have short half-lives, it has no known use outside from basic scientific study.
- The only applications for element are scientific investigation into its characteristics and the creation of other heavy atomic nuclei.
- Some of the chemical characteristics of mendelevium in aqueous solution have been explained with the aid of the isotope 256-Md.
- There is no biological use for mendelevium in living things.
Health Effects of Mendelevium
- The fact that mendelevium is a rare element means that there isn’t much information on its toxicity. However, it is radioactive and safer to assume its toxic.
- Because of its radioactive nature it can be absorbed if consumed, eventually settling in bones, lungs, liver, and testicles where it decays into other, potentially more hazardous radioactive elements.
Environmental Effects of Mendelevium
- Mendelevium doesn’t exist naturally and has not been identified in the earth’s crust, hence there is no reason to be worried about its environmental concerns.
Video Reference
References
- https://www.academia.edu/11041622/Fermium_Mendelevium_Nobelium_and_Lawrencium
- https://www.chemistrylearner.com/mendelevium.html
- https://www.rsc.org/periodic-table/element/101/mendelevium
- https://www.chemicool.com/elements/mendelevium.html
- https://www.thoughtco.com/mendelevium-facts-4126518
- https://periodic-table.com/mendelevium/
- https://chemicalengineeringworld.com/mendelevium-element-properties-and-information/

