When a material is combined with a solvent, various outcomes are possible. The substance’s solubility, which is defined as the solute’s highest achievable concentration, is what determines the outcome. The solubility rules aid in determining which compounds are soluble and to what amount. Some substances or solutes will dissolve, while others will produce a precipitate or solid, and only a handful will interact with water. Solubility rules can help you predict the ultimate states of materials when you’re working on chemical equations or developing a hypothesis.
Solubility rules are used to anticipate the solubility of ionic salts in water. They are necessary for multiple laboratory procedures as well as the preparation of pharmaceuticals.
Interesting Science Videos
Effects of Solubility on Reactions
There are three possible outcomes depending on a solute’s solubility:
- A solution that is diluted contains less solute than the maximum quantity it can dissolve (its solubility).
- It is said to be saturated if the amount of solute is exactly the same as its solubility.
- When there is more solute than can be dissolved, the surplus solute separates from the solution.
If the separation process includes crystallization, a precipitate is formed. Precipitation reduces the concentration of the solute to saturation, increasing the solution’s stability.
How to Use Solubility Rules
The sequence in which the solubility rules on this list are followed is very significant because, in the event that two regulations appear to be in conflict, you must abide by the first rule.
The data acquired from testing the solubility of several salts shows a variety of trends. These patterns serve as the foundation for the criteria stated in the table below, which can help forecast whether a certain salt will dissolve in water. These guidelines are based on the definitions of the terms soluble, insoluble, and somewhat soluble given below.
- Group I element salts (Li+, Na+, K+, Cs+, and Rb+) are soluble. The few exceptions to this rule are rare. Ammonium ion (NH4+) salts are also soluble in water.
- Nitrate ion (NO3–) salts are typically soluble.
- Salts with Cl–, Br–, or I– are usually soluble. Ag+, Pb2+, and (Hg2)2+ halide salts are prominent exceptions to this rule. As a result, AgCl, PbBr2, and Hg2Cl2 are insoluble.
- The vast majority of silver salts are insoluble. The most frequent soluble silver salts are AgNO3 and Ag(C2H3O2); almost all others are insoluble.
- The majority of sulfate salts are water-soluble. CaSO4, BaSO4, PbSO4, Ag2SO4, and SrSO4 are notable exceptions.
- Most hydroxide salts are only marginally soluble. Hydroxide salts of Group I elements are soluble. Hydroxide salts of Group II elements (Ca, Sr, and Ba) are weakly soluble. Transition metal and Al3+ hydroxide salts are insoluble. As a result, Fe(OH)3, Al(OH)3, and Co(OH)2 are not soluble.
- The majority of transition metal sulfides, including CdS, FeS, ZnS, and Ag2S, are exceptionally insoluble. Arsenic, antimony, bismuth, and lead sulfides are likewise insoluble.
- Carbonates are often insoluble. Group II carbonates (CaCO3, SrCO3, and BaCO3), as well as FeCO3 and PbCO3, are insoluble.
- Chromates are often insoluble. PbCrO4 and BaCrO4 are two examples.
- Ca3(PO4)2 and Ag3PO4 are typically insoluble phosphates.
- Fluorides like BaF2, MgF2, and PbF2 are often insoluble.
Solubility Rules Tabulated
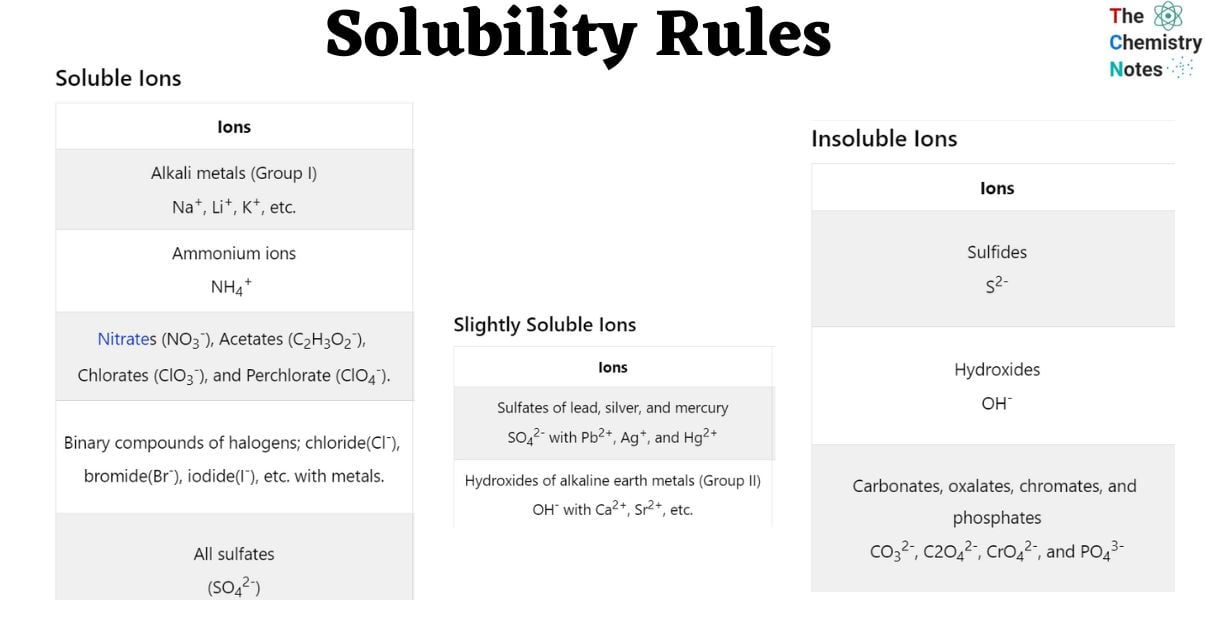
Soluble Ions
| Ions | Exception |
|---|---|
| Alkali metals (Group I) Na+, Li+, K+, etc. | |
| Ammonium ions NH4+ | |
| Nitrates (NO3–), Acetates (C2H3O2–), Chlorates (ClO3–), and Perchlorate (ClO4–). | |
| Binary compounds of halogens; chloride(Cl–), bromide(Br–), iodide(I–), etc. with metals. | Fluoride (F–), Silver (Ag) (Ag+), lead (Pb2+), and Mercury ( Hg2+). *Lead halides are soluble in hot water. |
| All sulfates (SO42-) | Barium (Ba2+), Strontium (Sr2+), Calcium (Ca2+), lead (Pb2+), Silver (Ag+), and Mercury (Hg2+). |
Slightly Soluble Ions
| Ions | Exception |
|---|---|
| Sulfates of lead, silver, and mercury SO42- with Pb2+, Ag+, and Hg2+ | Lead sulfate is poorly soluble |
| Hydroxides of alkaline earth metals (Group II) OH– with Ca2+, Sr2+, etc. | Barium Ba2+ |
Insoluble Ions
| Ions | Exception |
|---|---|
| Sulfides S2- | Calcium, barium, strontium, magnesium, sodium, potassium, and ammonium Ca2+, Ba2+, Sr2+, Mg2+, Na+, K+, and NH4+ |
| Hydroxides OH– | Alkali metals (Group I) and ammonium Na+, K+, etc. Al3+, NH4+ |
| Carbonates, oxalates, chromates, and phosphates CO32-, C2O42-, CrO42-, and PO43- | Alkali metals (Group I) and ammonium Na+, K+, etc. NH4+ *Lithium phosphate is poorly soluble |
Factors That Affect Solubility
Temperature: Solubility tends to rise with temperature if the dissolving reaction is endothermic. As temperature rises, solubility tends to decline if the dissolution is exothermic. Since most solids and liquids dissolve by endothermic processes, solubility typically rises with temperature. With the exception of cyclodextrin, the solubility of organic substances almost invariably rises with temperature. The behavior of gases is more complex and unpredictable.
Phase: Phase affects solubility. Even though both calcite and aragonite are types of calcium carbonate (CaCO3), their solubilities are different.
Other species: Other species in a solution have an impact on solubility. The ligands, common ions, and ionic strength of the solution are among the variables.
Shape and size of the particles: As a particle approaches saturation, solubility tends to rise with increasing surface area. An individual piece is less soluble than a fine powder. It matters whether something is crystalline or amorphous. Usually, solubility decreases as order increases.
Pressure: The solubility of solids and liquids is somewhat influenced by pressure. Although it is frequently disregarded in most applications, calcium sulfate fouling of oil wells, which happens in petroleum chemistry, is significant. The solubility of calcium sulfate reduces as pressure is reduced.
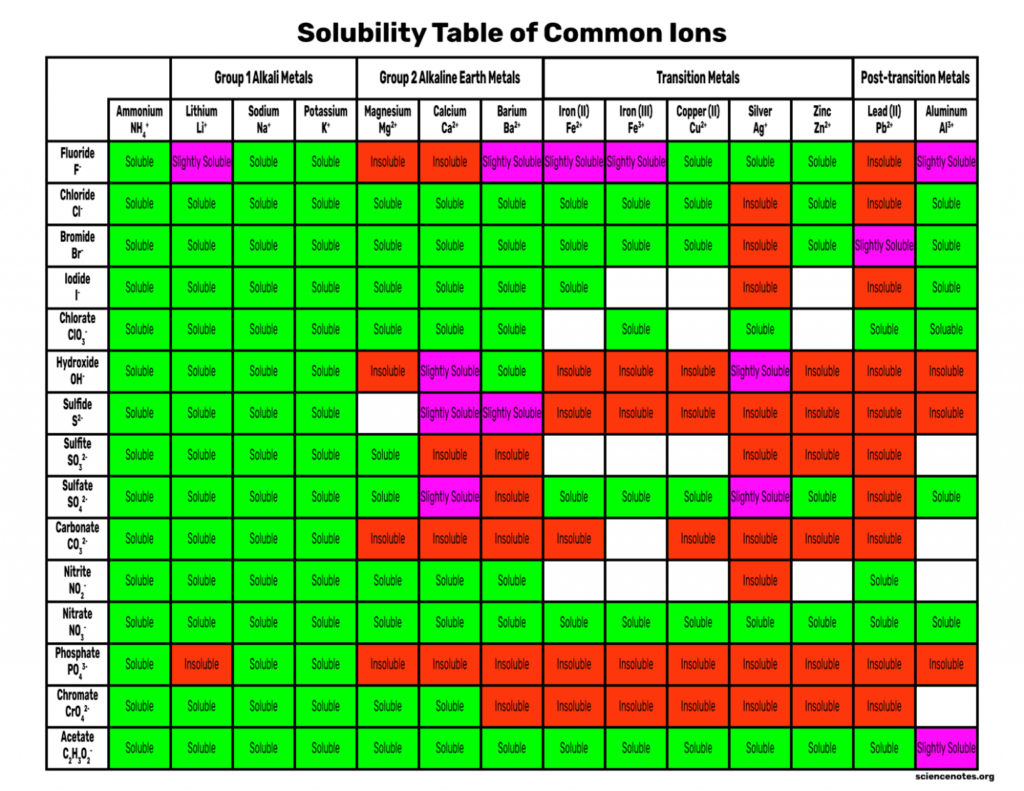
Solubility Rules Chart of Common Ions
Video References on Solubility Rules
References of Solubility Rules
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Equilibria/Solubilty/Solubility_Rules
- https://blog.prepscholar.com/solubility-rules-chart-chemistry
- https://chemistrytalk.org/solubility-rules-chart/
- https://www.chem.tamu.edu/rgroup/hughbanks/courses/462/handouts/The_Solubility_Rules.pdf
- https://psiberg.com/solubility-rules/
- https://chemed.chem.purdue.edu/genchem/topicreview/bp/ch18/soluble.php
- https://dept.harpercollege.edu/chemistry/chm/100/dgodambe/thedisk/chemsoln/solrules.htm

