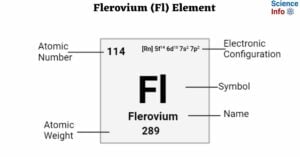
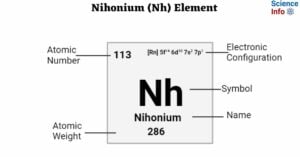
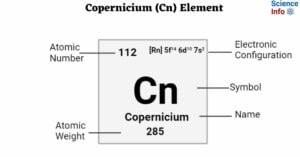
Livermorium (Lv) Element: Important Properties, Discovery, Uses, Effects
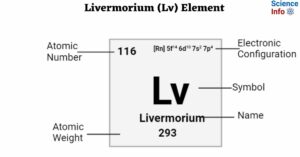
Livermorium is a synthetic transition metal with an atomic number of 116 and is represented by the symbol ‘Lv’ in the periodic table. It is silvery in appearance and belongs to the p-block of period 7 of the periodic table. Only … Read more