The field of chemistry includes the study of chemical entities, namely atoms and molecules, as well as the diverse manifestations of matter, including solid, liquid, and gaseous states. Chemistry is widely recognized as a pivotal scientific discipline due to its inherent interconnectedness with various fields, including Geology, Biology, Environmental Science, and Physics. Due to the inherent challenges associated with elucidating the interplay between two atoms or molecules, the utilization of chemical reactions and formulas serves as a valuable tool in enhancing our comprehension of such relationships.
Interesting Science Videos
Definition of Chemistry
“The branch of science that deals with the identification of the substances of which matter is composed; the investigation of their properties and the ways in which they interact, combine, and change; and the use of these processes to form new substances“– Oxford Dictionary
Chemistry is a branch of physical science that is primarily concerned with the comprehensive investigation of substances, encompassing both elements and compounds. Its primary objective is to examine and analyze the properties, behavior, structures, and practical applications of these substances.
While early civilizations had a basic comprehension of the differentiation between chemicals and compounds, the formal field of chemistry came into existence during the period known as the Age of Enlightenment. At this particular juncture, scientific methodologies were developed, incorporating the principles of empiricism and rationalism, in contrast to the dependence on supernaturalistic concepts.
Over the course of time, the field of chemistry has progressively evolved into a distinct and specialized scientific discipline, characterized by its various branches.
Importance of Chemistry
- The field of chemistry has existed since the inception of elemental formation within stellar furnaces. When stars undergo cataclysmic explosions known as supernovae, they disperse essential elements into space, thereby contributing to the enrichment of celestial bodies such as planets, which are crucial for the sustenance of life.
- Chemistry, as a fundamental process, plays a pivotal role in the functioning of the universe, encompassing a wide range of phenomena from nuclear reactions occurring within stars to intricate biochemical processes within living organisms. This intricate interplay of chemical reactions orchestrates the functioning of the universe, akin to sophisticated machinery.
- The scientific discipline of chemistry enables a thorough understanding of the universe and the human condition.
- The domain of technology encompasses a diverse array of applications that play a role in facilitating various facets of our everyday existence, encompassing areas such as sustenance, clothing, electronic devices, transportation, and pharmaceuticals, among others.
Branches of Chemistry
Chemistry, originating from the Egyptian term kēme (chem), denoting “earth,” is a scientific discipline that investigates the constitution, arrangement, and characteristics of substances, as well as the transformations they experience in the course of chemical reactions. Chemistry is commonly recognized as a fundamental scientific discipline due to its pivotal role in establishing connections between the physical sciences, including chemistry itself, and the realms of life sciences and applied sciences, such as medicine and engineering.
Chemistry is classified into various branches, namely:
Inorganic Chemistry
The field of chemistry that investigates compounds devoid of carbon and hydrogen atoms is commonly referred to as “inorganic chemistry.” In essence, it represents the antithesis of organic chemistry. Substances devoid of carbon-hydrogen bonds encompass metallic elements, inorganic salts, and various chemical compounds.
Organic Chemistry
Organic chemistry is the scientific field that encompasses the investigation of the structural characteristics, compositional makeup, and chemical attributes of organic compounds. The field of inquiry pertains to the examination of carbon and its various compounds.
Biochemistry
Biochemistry is a discipline within the realm of chemistry that delves into the intricate chemical processes occurring within living organisms and their interrelationships. This discipline is a laboratory-centric scientific field that establishes a connection between the realms of biology and chemistry. Through the application of chemical expertise and advanced technological tools, biochemists possess the capacity to comprehensively comprehend and effectively address intricate biological predicaments.
Physical Chemistry
The field of chemistry encompasses the study of both macroscopic and physical phenomena within the universe. The influence of physical properties on the chemical properties and structural characteristics of a substance is typically observed.
Analytical Chemistry
Analytical chemistry is the scientific discipline that employs sophisticated instrumentation and advanced analytical techniques to ascertain the structure, functionality, and properties of a given substance.
Matter
Matter is characterized as any entity possessing mass and occupying a specific volume within a given space. The three states of matter undergo transformations due to alterations in temperature and pressure conditions.
Generally, matter is commonly categorized into three distinct phases:
Gas
A gas is a substance that exhibits properties that are distinguished by the lack of a precisely defined volume and shape. Gaseous substances commonly exhibit the characteristic of occupying the entire volume of the container in which they are confined. As an illustrative example, one may consider the elemental constituents of Hydrogen (H) and Oxygen(O).
Liquid
A liquid is a substance that typically conforms to the shape of its container while maintaining a constant volume. Furthermore, it is worth noting that liquids possess the inherent characteristic of fluidity, allowing them to effortlessly flow or be poured. For instance, examples of liquids include water, milk, oil, mercury, and alcohol among others.
Solid
A solid is a substance that exhibits a clearly defined geometric structure and maintains its volume in accordance with its specific shape. Moreover, it is worth noting that solids demonstrate a restricted range of molecular mobility. For instance, substances, namely Sucrose, Iron, gold, and lignocellulose, serve as prime examples of a diverse array of materials that are frequently encountered in various contexts.
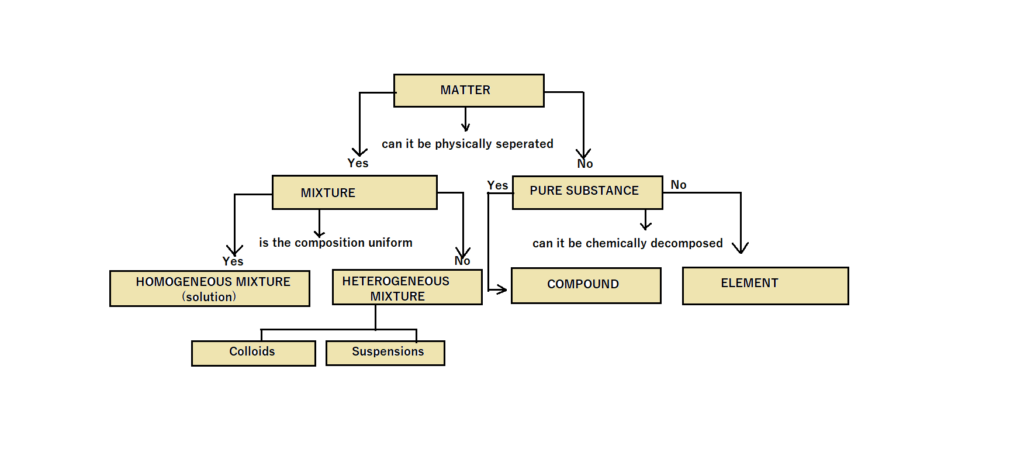
Classification of Matter
Matter can be further divided into
(I) Mixtures
A Mixture can be defined as a composite material comprising multiple discernible substances that coexist in various ratios, without any explicit constraints on their relative quantities. It is imperative to recognize that mixtures can be classified into two distinct categories, namely heterogeneous mixtures and homogeneous mixtures.
Homogeneous Mixture
Two substances are combined to create a Homogeneous Mixture, meaning that the composition of the substances is consistent throughout the mixture. Sugar solution and air are examples of homogeneous mixtures.
Heterogeneous Mixture
The amalgamation of multiple substances results in a Heterogeneous Mixture, distinguished by its non-uniform composition throughout. Numerous occurrences of suspensions can be discerned, exemplified by the confluence of two solid entities, specifically Sodium Chloride (NaCl), and sucrose.
(II) Pure Substance
A substance composed exclusively of particles of identical nature is commonly denoted as a pure substance. Within the domain of chemistry, it is duly noted that diverse manifestations of matter consistently display a resolute chemical composition and maintain unalterable chemical characteristics. Moreover, these entities exhibit inherent resistance to disassembly into their fundamental components via physical mechanisms. Pure substances can be further categorized into the subsequent classifications:
Element
An element is precisely characterized as a homogenous substance consisting solely of a singular type of atom, thereby rendering it impervious to any subsequent decomposition. The constituent components are further categorized into three distinct classifications according to their inherent physical and chemical attributes, namely: (1) Metals, (2) Non-metals, and (3) Metalloids.
Compound
A Chemical Compound can be defined as a substance that is formed through the precise combination of multiple elements, ensuring a specific mass ratio is maintained. Furthermore, it is important to note that the properties of a compound are inherently distinct and discernible from those displayed by its individual constituent elements. Moreover, it is crucial to acknowledge that the constituents constituting a compound possess an inherent inability to be deconstructed into more fundamental substances via the application of physical methodologies. The exclusive and effective approach for the segregation of these entities is achieved by employing chemical methodologies.
Properties of Matter
All substances possess unique and inherent properties. In scientific inquiry, properties are commonly classified into two categories: Physical Properties and Chemical Properties.
Physical Properties
Physical properties are characteristics that can be quantified or observed without altering the fundamental identity or composition of a substance. Examples of physical qualities include color, odor, melting point, Boiling Point, and density, among others.
Chemical Properties
Chemical properties encompass the distinctive attributes exhibited by particular substances, which manifest themselves through discernible transformations occurring during chemical reactions. Several prominent chemical properties encompass flammability, toxicity, heat of combustion, pH, radioactive decay rate, and chemical stability.
Nature of Matter
| Property | Solid | Liquid | Gas |
|---|---|---|---|
| Intermolecular Space | Minium | Intermediate | Minimum |
| Force of Attraction | Maximum | Intermediate | Maximum |
| Density | Maximum | Intermediate | Maximum |
| Shape | Fixed | Variable | Variable |
| Kinetic Energy | Minimum | Intermediate | Maximum |
| Volume | Fixed | Fixed | Variable |
| Compressibility | Minimum | Intermediate | Maximum |
Mass and Weight
The quantity of matter contained within a substance is referred to as its mass, while the gravitational force acting upon an object is known as its weight.
The conservation of mass dictates that the quantity of matter remains constant, while variations in gravitational force give rise to fluctuations in its weight across different locations.
The kilogram (kg) is recognized as the standard unit of mass in the International System of Units (SI). The Newton is the SI-derived unit of weight, which serves as the derived unit of the SI base unit.
Volume
Volume can be defined as the quantification of three-dimensional space that is enclosed by specific closed boundaries. This encompasses the spatial extent occupied or encompassed by a given substance, such as a solid, liquid, gas, or plasma, as well as various shapes. The quantification of volume is commonly achieved through the utilization of numerical values expressed in SI-derived units, specifically cubic meters.
Density
The concept of mass density, also referred to as density, pertains to the quantification of a material’s mass in relation to its volume. The variable denoting density is conventionally represented by the Greek letter ρ, pronounced: “rho”. The internationally recognized unit for measuring density is the kilogram per cubic meter (kg/m3).
Temperature
Temperature is a fundamental physical attribute inherent to matter, serving as a quantitative measure that encapsulates the abstract notions of thermal energy and its absence. Three widely used scales for quantifying temperature are Celsius, Fahrenheit, and Kelvin. The interrelation between the temperature measurements on two distinct scales can be expressed through the subsequent mathematical relationship:
Fahrenheit (∘F) = 9/5 Celsius (∘C) + 32 Kelvin (K)
Kelvin (K) = Celsius (∘C) + 273.15
Law of Chemical Combination
Law of Conservation of Mass
In a chemical reaction, the mass of reactants consumed is equal to the mass of the products formed, indicating the conservation of mass. According to the fundamental principle of this law, matter has an inherent property that prevents its generation or annihilation. This concept can be defined as Stoichiometry, which states that the amount of reactants used in a chemical reaction is directly proportional to the number of products produced. This is a result of the law of conservation of atoms. Antoine Lavoisier defined this law in 1789.
Law of Definite Proportion
The Law of definite proportions asserts that the mass ratios of elements within a composite sample remain constant. According to this law, compounds consistently maintain a fixed ratio by mass of their constituent elements, regardless of their origin or method of formation. The discovery is credited to Joseph Proust, a chemist from France.
Law of Multiple Proportions
This law proposes that in the process of chemical combination when two elements unite to produce one or more compounds, the masses of one element that combine with a given mass of the other element will always be in a ratio of simple, whole numbers. The law of multiple proportions was proposed by Dalton in the year 1803.
Law of Reciprocal Proportions
According to the law, when two distinct elements are mixed with a constant mass of a third element, the ratio of their combined masses remains consistent or can be expressed as a straightforward multiple of their combined mass. The Law of reciprocal proportions was introduced by Richter in the year 1792.
Gay Lussac’s Law of Gaseous Volume
The law proposed by Gay Lussac in 1808 states that there exists a direct relationship between the volume of a gaseous reactant and the resulting product. This relationship can be accurately represented as a whole number ratio.
Avogardo Law
In 1811, Avogadro proposed the idea that gases, when at the same temperature and pressure, would contain the same amount of molecules if they occupied the same volume. This law is known as Avogadro’s Law.
Dalton’s Atomic Theory
In 1808, Dalton published a book titled ‘A New System of Chemical Philosophy, in which he presented Dalton’s Atomic Theory:
- Matter is composed of atoms that cannot be divided.
- All atoms of a specific element possess identical properties, including having the same mass. Atoms of different elements have varying masses.
- Compounds are created when atoms from distinct elements combine in a specific ratio.
- Chemical reactions involve the reorganization of atoms. In a chemical reaction, these substances are neither created nor destroyed.
Atoms
- The atom is widely regarded as the fundamental and elemental particle that plays a pivotal role in determining the unique characteristics and properties of chemical substances.
- The central region of an atom, known as the nucleus, is composed of positively charged particles called protons and electrically neutral particles called neutrons.
- The categorization of an element is determined by its proton count, while the combination of protons and neutrons determines its atomic mass.
- The central region of an atom, known as the nucleus, is encompassed by a collection of electrons that occupy distinct orbitals.
- The elemental atoms react with each other to form molecules, which then come together to create various macroscopic objects, including living organisms.
Mass of Atom
There exist two methods for indicating the mass of atoms, which encompass:
First Method
- The idea of atomic mass is a fundamental principle within the field of chemistry, denoting the mass of a singular atom. The measurement of this quantity is commonly expressed in atomic mass units (amu) or unified mass (u).
- The atomic mass unit (a.m.u.) is precisely defined as being equivalent to one-twelfth of the mass of a single carbon-12 (C12) atom.
- i.e. 1 a.m.u = (1/12)th of the mass of C12 atom
Second Method
- The mass of 6.022 × 1023 atoms of a particular element is measured in grams. This is alternatively referred to as the molar atomic mass.
- A mole is a fundamental unit of measurement in chemistry and physics.
- i.e. 1 dozen = 12
- 1 million = 106
- 1 mole = 6. 022 x 1023
- The mass of a single atom, expressed in atomic mass units (amu), is numerically equivalent to the mass of 6.022 × 1023 atoms, expressed in grams.
- The term “molar atomic mass” refers to the atomic mass expressed in grams.
- The quantity 6.022 × 1023 is commonly referred to as 1 mole of atoms, and it is also known as Avogadro’s Number.
Molecules
- A molecule can be described as the exquisite outcome of a harmonious fusion between two or more atoms, wherein they establish a chemical bond, be it ionic or covalent in nature.
- The smallest particle, referred to as the fundamental unit, encompasses all the inherent physical and chemical attributes specific to a given substance. A molecule consists of one or more atoms. For example, the chemical compounds dihydrogen (H2) and Ammonia (NH3) can be regarded as illustrative instances.
- Molecules can be composed of elements that exhibit an intriguing pattern, as demonstrated by the seven diatomic elements: hydrogen (H), nitrogen (N), oxygen (O), fluorine (F), chlorine (Cl), bromine (Br), and iodine (I).
- The calculation of a molecule’s mass entails the aggregation of the individual masses of its constituent atoms. Therefore, it is important to acknowledge that the quantification of a molecule’s mass can be achieved through two commonly utilized methods for measuring atomic mass: atomic mass units (amu) and grams per mole (g/mol).
Empirical Formula and Molecular Formula
- The empirical formula refers to the most basic integer ratio of atoms found in a given molecule, while the molecular formula denotes the precise count of atoms present in the molecule.
- As an illustration, glucose specifically denoted as C6H12O6, possesses a molecular formula of C6H12O6, while its empirical formula is CH2O.
The mathematical expression that describes the relationship between empirical formula mass and molecular formula mass is as follows: - n= Molecular mass / empirical formula mass
- Here, n is the simplest ratio.
Stoichiometry and Stoichiometric Calculations
This concept facilitates the determination of the mass or quantity of reactants and products involved in a specific chemical reaction. In order to perform calculations, it is imperative to establish a balanced chemical equation as a prerequisite. This step is essential as it enables the subsequent prediction of the masses of both reactants and products. As an illustration,
The chemical reaction is expressed in the following manner:
2 H2 + O2 → 2 H2O
The chemical equation provided exhibits balance, indicating that the reaction entails the amalgamation of one mole of oxygen with two moles of hydrogen to yield two moles of water. Therefore, it is possible to proceed with the determination of the masses of the individual elements involved.
Limiting Reagent (LR) and Excess Reagent (ER)
- If the reagents are not used in a stoichiometric way, the amount of product made is limited by the reagent that is not used enough. This reagent is called the “limiting reagent.” Conversely, the reagent that exceeds the requisite quantity is referred to as the excess reagent.
- As an illustration, in the event that we engage in the combustion of carbon within the atmosphere, wherein an abundant reservoir of oxygen is readily available, the quantity of carbon dioxide generated shall be contingent upon the magnitude of carbon assimilated.
- In this particular scenario, it can be discerned that carbon serves as the limiting reactant, while oxygen, denoted as O2, assumes the role of the excess reactant.
Percent Yield
- As previously stated, practical limitations are the main reason why the observed quantity of the chemical reaction’s product is lower than the predicted value.
- The percentage yield is calculated by multiplying the discrepancy between the actual and predicted quantities of the product.
The Reaction of Aqueous Media
- The two solids are incapable of undergoing a chemical reaction while in the solid phase, thus necessitating their dissolution in a liquid medium. When solutes are dissolved in a solvent, they exist together in a homogeneous mixture known as a solution.
- Multiple parameters are employed to assess the efficacy of the solution. The concentration of a solution refers to the quantity of solute present within the solution.
- The parameters employed to indicate the potency of a solution are:
- Mole fraction X: moles of a component / Total moles of solution
- Mass%: Mass of solute (in g) present in 100g of solution
- Mass/Vol: Mass of solute (in g) present in 100mL of solution
- v/v: Volume of solute/volume of solution (only for liquid-liquid solutions)
- g/L: Wt. of solute (g) in1L of solution
- ppm:( mass of solute/mass of solution) x 10
- Molarity (M): moles of solute/Volume of solution (L)
- Molality (m): moles of solute/mass of solvent (Kg)
Important Relation
(i) Solution chemistry includes the link between molality (m), molarity (M), density (d), and solute molar mass (MO). Molality is the number of moles of solute per kilogram of solvent. However, molarity is the number of moles of solute per liter of solution. Molality and molarity are related by solution density. Mass per volume is density. Solution-wise, it means
d: density in g/mL
Mo: molar mass in gmol1
molality, m= (M x 1000)/(1000d - M Mo)(ii) Relationship between molality (m) and mole fraction (XB) of the solute
m = (XB/(1-XB)) x (1000/ MX)m = ((1-XA)/XA) x (1000/MA)Notes:
- Molarity is widely recognized as the predominant unit of measurement for quantifying the concentration of a solution.
- The number of moles of solute, represented as n, can be calculated by multiplying the molarity (M) of the solution by its volume (V), as indicated by the equation n = M × V.
- In all formulas pertaining to strength, the numerator consistently incorporates a quantification of the solute, expressed either in terms of mass or moles.
- Except for molality (m), all the formulas in question feature a solution quantity in the denominator.
Dilution Law
- The number of moles in a solution remains constant during the process of dilution. If the initial volume of a solution with a given molarity (M1) is diluted by adding additional solvent, resulting in a final volume of V2, the relationship can be expressed as follows:
The equation M1V1 = moles of solute in the solution = M2V2 - It represents the relationship between the concentration and volume of a solute in a solution.
Effect of Temperature
- The observed correlation between temperature and solvent volume is indicative of a temperature-dependent relationship, wherein an increase in temperature is accompanied by a corresponding increase in solvent volume.
- In the context of a closed system, wherein there is no mass loss and the quality of the solute within the solution remains unaffected, it is pertinent to consider the following scenario.
- The formulas pertaining to the strength of solutions, specifically those that do not incorporate the volume of the solution, remain unaltered in the face of temperature variations.
- For instance, the molality of a solution does not vary with changes in temperature. Formulas pertaining to volume are subject to modification as a result of temperature variations, such as in the case of molarity.
Chemical Compound
- Chemical compounds are typically classified based on the nature of the bonds that unite the atoms and give rise to the formation of molecules. Sugars and salts, respectively, are examples of compounds that can exhibit either ionic or covalent bonding.
- Organic compounds are characterized by the presence of carbon as the primary constituent, serving as the backbone atom. Conversely, inorganic compounds may also incorporate carbon atoms, but they do not necessarily occupy a central role within the compound’s structure.
Periodic Table
- The periodic table of chemical elements serves as a comprehensive and methodical compilation of knowledge pertaining to the elements, meticulously arranged according to their periodic patterns.
- Chemical elements are systematically organized based on their group properties, such as in the case of noble gases. Every entry within the dataset comprises essential details, encompassing the atomic number, atomic weight, electronic configuration, and electronegativity.
Acids and Bases
- Acids and bases exhibit contrasting chemical properties as reflected by their divergent pH values.
- In the context of a logarithmic scale ranging from 0 to 14, it is observed that substances with acidic properties exhibit pH values below 7, while substances with basic properties exhibit pH values above 7. There exist three theoretical frameworks that elucidate the functioning principles of acids and bases:
- The Arrhenius Theory
The Bronsted-Lowry Theory
The Lewis electron-pair Theory - In the context of neutralization reactions, it is typically observed that an acid acts as a proton donor. The process of chemical interaction between acids and bases is commonly referred to as neutralization, as it yields neutral salts and water as the final products.
Conjugation
- The phenomenon of conjugation in the realm of organic chemistry refers to the alignment and subsequent overlap of p-orbitals with delocalized electrons within a given molecule.
- The phenomenon of conjugation yields a diverse array of practical entities, namely conjugative polymers and carbon nanotubes.
Chemical Reactions
Chemical Reactions are systematically categorized based on the manner in which the constituents of substances engage and reconfigure themselves during the process of reacting with one another. The repertoire of chemical reactions commonly encountered encompasses:
Decomposition / Analysis Reaction
A decomposition reaction is the process of breaking up a substance into two or more simpler constituents with the help of heat, light, electricity, etc. The general form of the decomposition reaction, otherwise known as the analysis reaction, is:
XY → X + Y
Most of the decomposition reactions are endothermic.
Synthesis / Combination Reaction
The process of two or more substances reacting to form a new compound is known as a synthesis reaction. The general form of the synthesis reaction, otherwise known as the combination reaction, is:
X + Y → XY
Most of the synthesis reactions are exothermic.
Single Displacement / Replacement Reaction
Single displacement reactions are chemical reactions in which a more reactive element displaces a less reactive element from its aqueous salt solution. In these reactions, products can be determined through reactivity series. It is a series where elements are arranged in decreasing order of their reactivity. The general form of the single displacement reaction, otherwise known as the single replacement or substitution reaction, is:
X + YZ → XZ + Y
Double Displacement / Metathesis Reaction
In double displacement reactions, two aqueous ionic compounds exchange ions (mostly cations) to form completely different compounds. The exchange during this type of reaction is either of cations or anions. It’s never both at the same time.
The general form of the double displacement reaction, otherwise known as the metathesis, is:
XY + ZA → XZ + YA
Combustion Reaction
The most basic kinds of chemical reactions frequently include the combustion process. Compounds are burned and released in the form of heat and light energy. Oxygen is one of the necessary reactants in combustion reactions.
Acid-Base Reaction / Neutralization Reaction
An acid-base reaction is a type of double displacement reaction that occurs between an acid and a base. It is the process by which salt and water form when a reaction occurs between acid and base.
The general form of the acid-base reaction, otherwise known as the neutralization reaction, is:
HX + YOH → H2O + YX
The H+ ion in the acid reacts with the OH– ion in the base to form water and an ionic salt.
Hydrolysis Reaction
Hydrolysis is the term for any decomposition process that occurs with water action. Hydro means water, and lysis means to break down. In general, hydrolysis is the interaction of positive and negative ions of salt with water, resulting in acidity or basicity in the solution.
The general form of the hydrolysis reaction is:
XY + H2O → XH + YOH
Precipitation Reaction
It is the process of forming one of the products as an insoluble product by mixing two compounds from their aqueous solution. Precipitation reactions in aqueous solutions are mostly double-decomposition reactions. Like the neutralization reaction, it also qualifies as a double displacement.
References
- https://leverageedu.com/blog/some-basic-concepts-of-chemistry-class-11/
- https://www.chemicals.co.uk/blog/10-basic-concepts-of-chemistry
- https://chem.libretexts.org/Courses/University_of_Arkansas_Little_Rock/Chem_1402%3A_General_Chemistry_1_(Belford)/Text/1.A%3A_Basic_Concepts_of_Chemistry
- https://testbook.com/chemistry/basic-concepts-of-chemistry
- https://www.khanacademy.org/science/class-11-chemistry-india/xfbb6cb8fc2bd00c8:in-in-some-basic
- https://school.careers360.com/chemistry/some-basic-concepts-of-chemistry-chapter-pge
